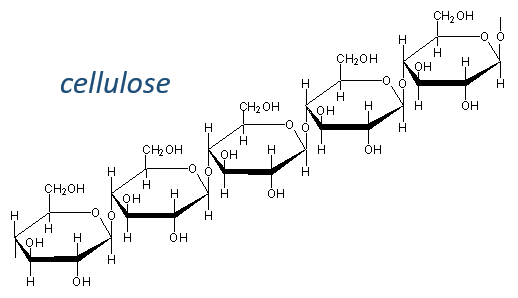
Les glucides sont essentiellement des hydrates cycliques de carbone (CN (H2O) N) mais peuvent aussi porter N, S, P. Les cycles ont habituellement une longueur de 5 ou 6 atomes et forment des macromolécules lorsque les cycles se lient. Un exemple est la cellulose, qui est présente dans les parois des légumes. En tant qu’êtres humains, nous ne digérons pas correctement la cellulose contenue dans les salades que nous mangeons, nous devons donc la mastiquer davantage pour la casser. Un autre exemple est la chitine qui est l’élément essentiel de la coquille des insectes, des crabes, etc.
La nomenclature des monocycles (ou des monomères dans lesquels nous considérons les macromolécules comme des polymères) est de terminer leur nom par ~ monosaccharide. Les plus connus sont le glucose et le fructose, qui diffèrent par la présence d’une cétone dans le fructose. Le glucose est le monosaccharide le plus abondant et représente 50% des carbones sur Terre. Les algues produisent des milliards de monosaccharides par année et nécessitent la lumière du soleil pour le faire. Dans l’industrie, nous utilisons des monosaccharides dans le papier, le coton, les boissons, la nourriture, les produits pharmaceutiques, …
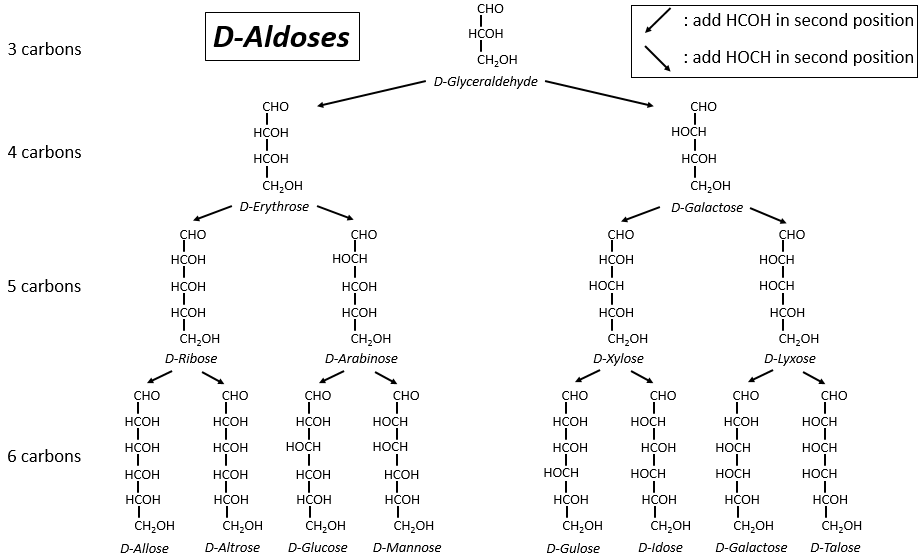
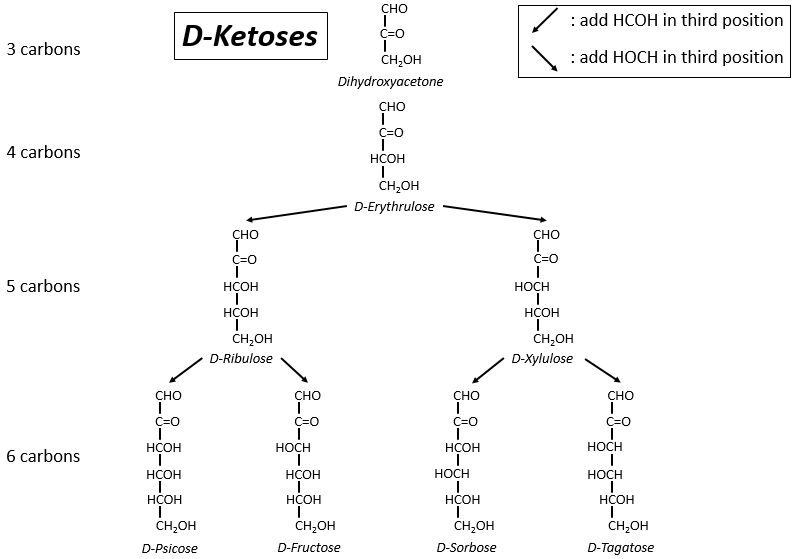
Les tableaux suivants montrent les projections de Fischer des sucres. Fondamentalement, les chaînes sont composées de carbones portant des fonctions -OH. A l’extrémité supérieure, il y a un aldéhyde dans le cas des aldoses et une cétone dans le cas des cétoses. C’est le premier paramètre de tri. Le deuxième paramètre est la longueur de la chaîne, allant de 3 à 8 (les chaînes de 8 atomes de carbone sont fabriquées artificiellement).
On a pu voir que les -OH ne sont pas tous du même côté de la chaîne. Les carbones à l’intérieur de la chaîne (pas ceux aux extrémités) sont chiraux et les sucres ont donc une activité optique. Les tableaux que nous avons montrés précédemment ne montrent que les molécules de dextrogyre, notées avec le préfixe D-, c’est-à-dire celles qui dévient la lumière vers la droite. La distinction n’est pas seulement importante pour la lumière: la plupart des enzymes n’acceptent qu’un seul des conformères, L (levogyre) ou D (dextrogyre).
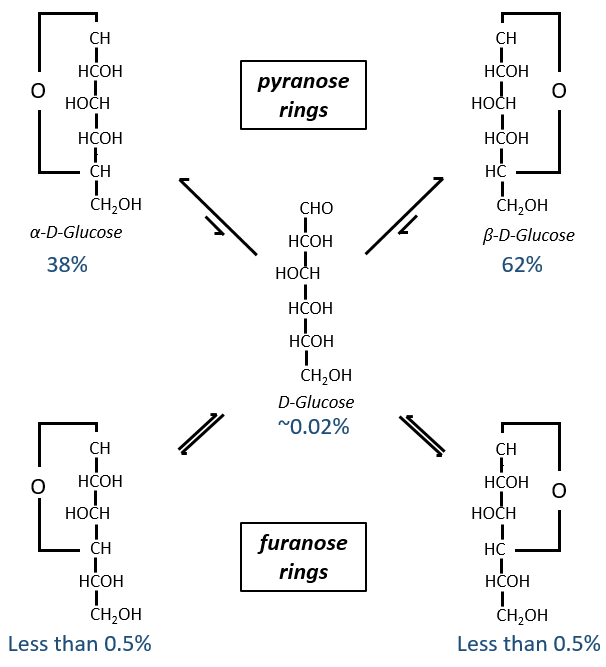
Maintenant vous me direz que j’ai commencé cette section en disant que les glucides sont cycliques et que je n’ai montré que des molécules linéaires. Dans des conditions aqueuses, l’aldéhyde et une fonction hydroxyle (en position 4 ou 5) fusionnent pour former un hémiacétal, un cycle de 5 (cycles de furanose) ou 6 atomes (cycles de pyranose).
The equilibrium of this process is almost towards the cyclic forms with 6 atoms, i.e. the pyranose rings. The crystals of the linear sugars were obtained in pyridine in which the rotatory power is +19° for the glucose and in the water it was +53°.
This rotatory power is the averaged value of the species in the water (the α-D-glucose has [α]D=120 and the β-D-glucose has [α]D=19).
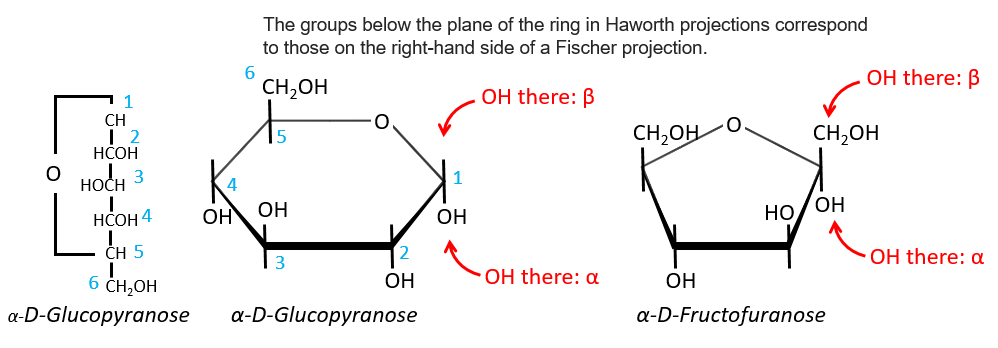
Projection of Haworth
The cycle is represented flat. The heterocyclic oxygen is on the top right position and the chain outside of the cycle is on top left position of the cycle. The –OH groups are placed on top of the cycle if they are on the left in the projection of Fischer and under the cycle if they are on the right of the chain in the projection of Fischer.
We enumerate the carbons starting from the right and moving in the clockwise direction. The C1 is the highest carbon of the Fischer projection and is here an anomeric carbon: when the glucose was put in water, the OH group could be on the left or right of the chain. Its position determines if the cycle is α or β and we can pass from the α to the β forms only by the linear form. We have the same kind of representation for furanose rings and the cyclisation is also done in the case of ketoses and of pentoses. Also note that the C1 carbon may be out of the cycle, as it is the case for the α-D-fructofuranose shown above.
Osides are monosaccharides bound to one molecule on the anomeric spot. The presence of this molecule there blocks the configuration of the monosaccharide: it cannot change from α to β or change for its linear form anymore. As the anomeric carbon is not available, the reduction power of the monosaccharide is also lost.
Disaccharides
They are combinations of two monosaccharides. We will use them to show where the liaisons can be and how we represent those molecules in the representation of Haworth.
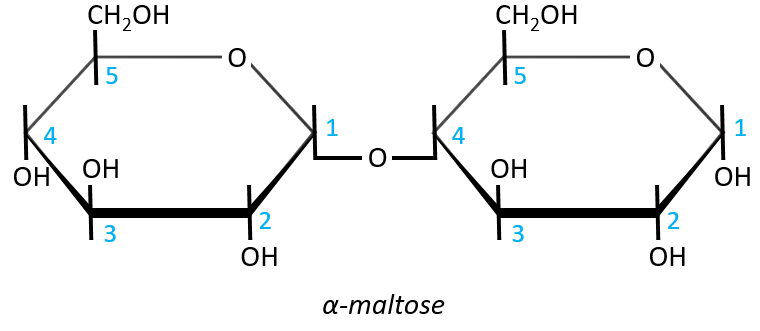
- Maltose
It is the combination of two glucoses bound together in (1→4).
The liaison is made between the C1 of the glucose of the left and the C4 of the glucose of the right, indicated by (1→4). We let the representation of both glucoses as they were for the monosaccharides alone and bind them with a straight line. It is as simple as that. One anomeric carbon is taken by the liaison but the second is free. The molecule is thus a reductant and able to do the mutarotation.
The nomenclature of the α-maltose is
α-D-Glucopyranosyl-(1→4)-D-glucose
or also
4-O-α-D-Glucopyranosyl-D-glucose
The monosaccharide with its anomeric carbon free ends the name while the other monosaccharide name is ended by ~osyl. The position of the liaison between the monosaccharides is indicated by the (1→4) written between the monosaccharides or the 4-O prefix: liaisons between monosaccharides are always involving C1 so we just indicate the second carbon involved in the liaison (C4) and the fact that the liaison involves and oxygen atom. The alpha also gives an information on the liaison between the sugars: the type of the anomeric carbon that is now bound to the second monosaccharide. The dextro or levogyre character of each monosaccharide is indicated as well.
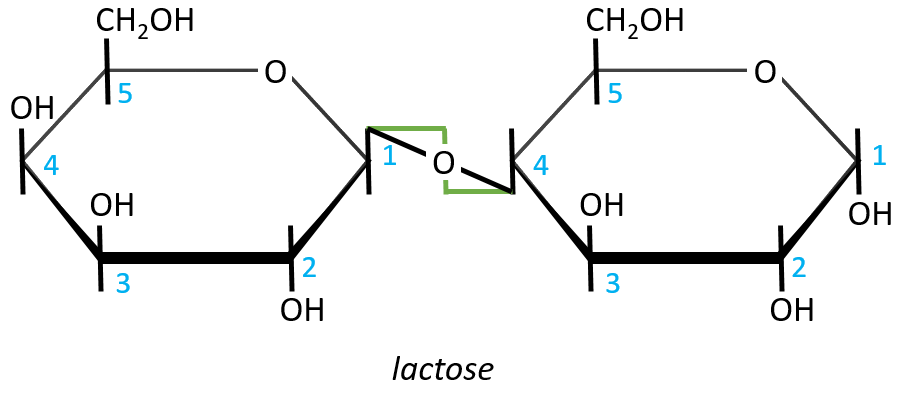
- Lactose
It is the combination of one β-galactose with one α-glucose. The liaison is also in (1→4).
In this representation, we maintain both representations of the monosaccharides. The liaison between them is thus represented as a diagonal or as a S shaped line as showed above in green. The nomenclature is
β-D-galactopyranosyl-(1→4)-D-glucose
or
4-O-β-D-galactopyranosyl-D-glucose
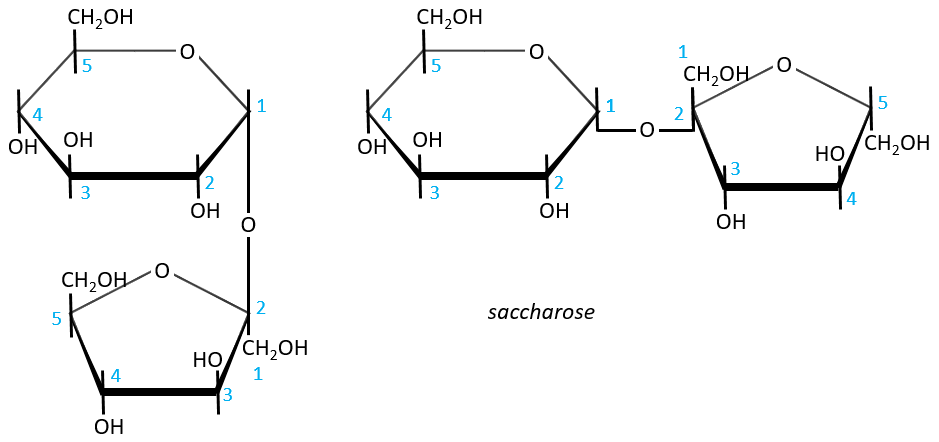
- Sucrose (or saccharose)
It is an example of disaccharide for which the liaison is not 1à4. The saccharose is the combination of a α-glucose with a β-fructose. They are bond by their anomeric carbons, i.e. in (1→2).
There is thus no possibility of mutarotation. The nomenclature is α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside. The name doesn’t end by ose because the anomeric carbon is not free.
The invertase is an enzyme that is able to cleave the liaison between those two monosaccharides. This enzyme is interesting for the industry because the saccharose has a sweetening power way smaller than the sum of the sweetening powers of the fructose and of the glucose.
Polysaccharides
They are chains of monosaccharides. We can sort them in two types: homopolyosides that are chains of one single monosaccharide that is repeated, and heteropolyosides that are (usually) two different monosaccharides that are repeated with a given order or at random. The size of the chain can be very long (thousands to hundreds of thousands of monosaccharides).
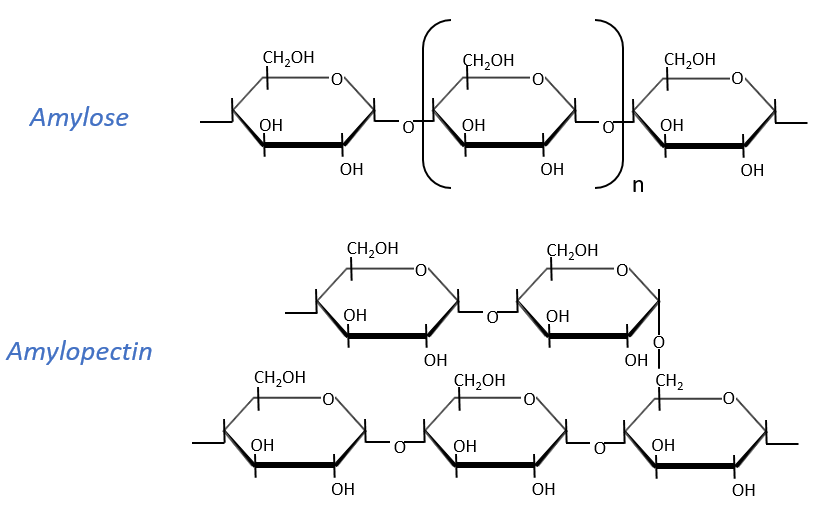
Starch is one macromolecule we find in vegetables, composed of monosaccharides. There are separated in two groups: the amylose (15-20%) and the amylopectin (80-85%). The amylose is a chain of glucoses linked in (1→4) 100 to 300 units long while the amylopectin has ramifications every 30 sugars approximatively, linked in (1→6). In animals, the equivalent of the amylopectin is the glycogen for which the ramifications are more frequent (every 8 to 12 sugar). The amylose forms a α helix (6 to 8 glucoses form one whorl).
Enzymes can cleave liaisons to free glucose molecules. Some enzymes cleave in the chains (endoglycosidases), some at their extremities (exoglycosidases). Given the size of the starch and the amount of extremities, several enzymes can act simultaneously.
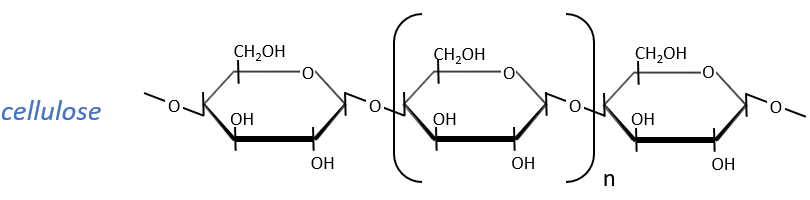
The cellulose is made of β-glucoses, up to 10000 units. This difference with the amylose leads to a huge difference of conformation: instead of helixes, the cellulose forms planes bound together by H bonds. Moreover, the β liaisons cannot be cleaved by the enzymes of most of the animals. Only ruminants are able to do it because they have cellulases, enzymes able to cleave the cellulose.
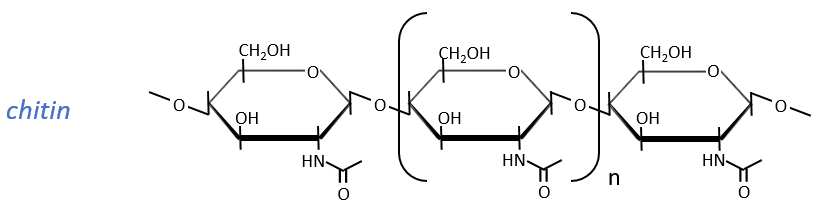
The chitin is similar to the cellulose except that the beta glucoses are replaced by a variant (N-acetylglucosamine). Combined to Ca2CO3 it forms exoskeletons of insects.
Heteropolyosides
Glycosaminoglycan
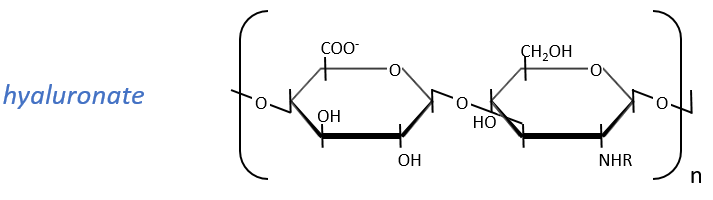
They are found at the outside of the cells, bound to the lipid bilayer and allow the movements of the cell (one motor is in the lipid bilayer). An example is the hyaluronic acid (the cosmetics industrials highlight it nowadays).
This molecule is a polymer composed of a repetition of a block of two monosaccharides, the β-glucoronate and one N-acetylglucosamine. They are bound in (1→4) and in (1→3) to form one chain. As the β-glucoronate is negatively charged, the chains don’t interfere one with each other. Chains are bound to proteins
Peptidoglycans
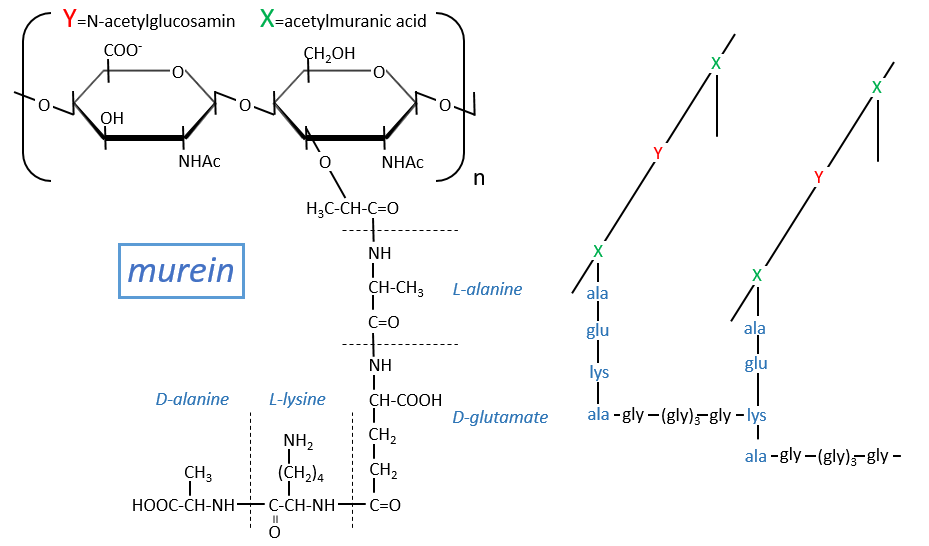
Some monosaccharides of the chain wear a peptide chain of amino acids. The peptide chains bind together to form a network with a given rigidity. Peptidoglycans are also known as the murein.
It is the rigid constituent of the membrane of bacteria’s. It is composed of a block of N-acetylglucosyl (a derivative of the glucose) bound in (1→4) with the N-acetylmuranic acid, the same monosaccharide but wearing one peptide chain on C3. The chain is bound to another chain by a liaison between several glycine’s and lysine to form a dense network. The peptides in the chains are consecutively L, D, L, D, …
The mechanism of some antibiotics is to break the liaison between the monosaccharides to destroy the whole structure.