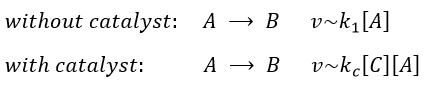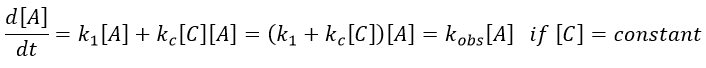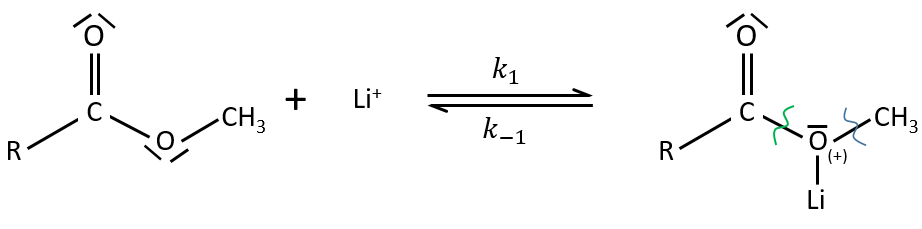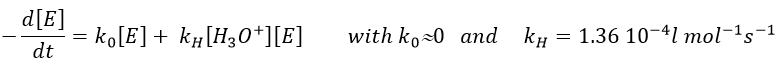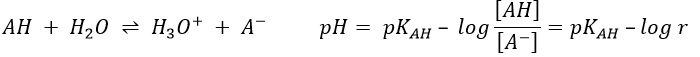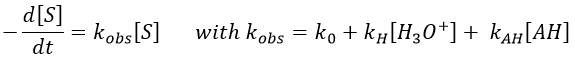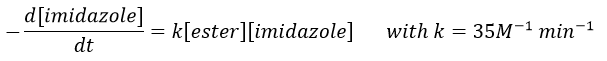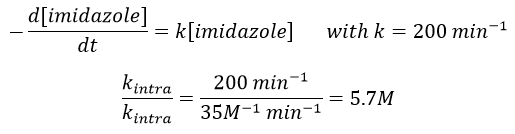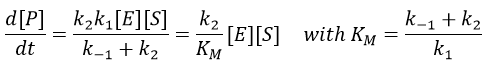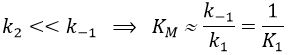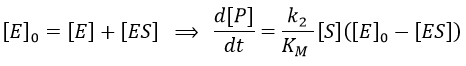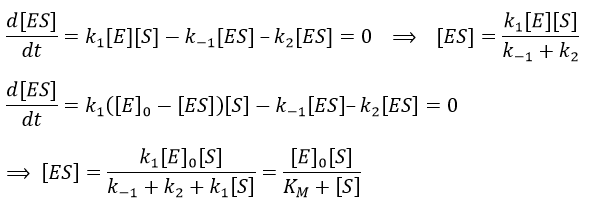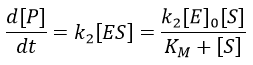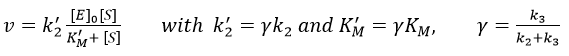Homogeneous catalysis
In some conditions, a same reaction A → B can lead to two different kinetics depending on the composition of the solution in which it is. One species C, apparently not intervening in the reaction as it is not consumed by it, increases the speed of reaction.
Both reactions occur simultaneously, giving a global reaction speed of
The ratio between the two paths is given by kc[C]/k1. The concentration of the catalyst can thus affect the contribution of the reaction paths. If the product of the reaction is the catalyst, then we say that the reaction is autocatalytic: the presence of the product enhances its own formation.
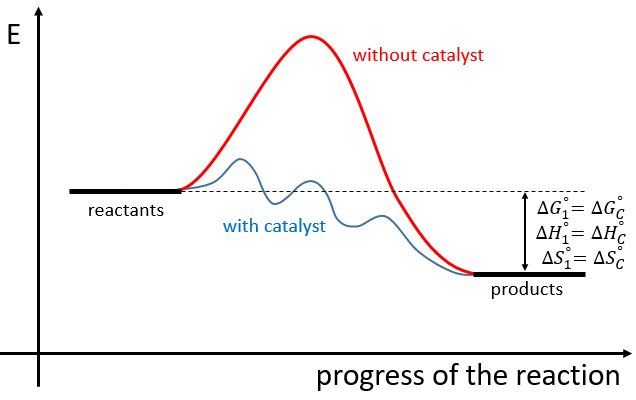
The use of a catalyst doesn’t change the difference of enthalpy and entropy between the products and the reactants but only the reaction path. The catalysed reactions involves several steps of smaller energy of activation than the normal reaction
If the reaction is a reaction of equilibrium, the catalyst is catalyst of both sides of the reaction.
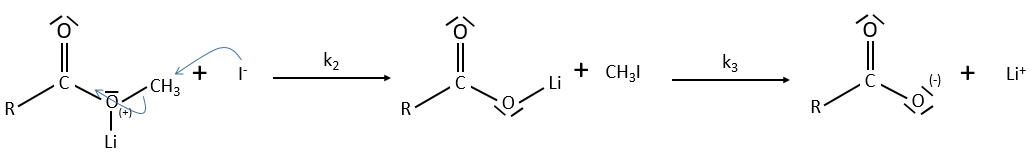
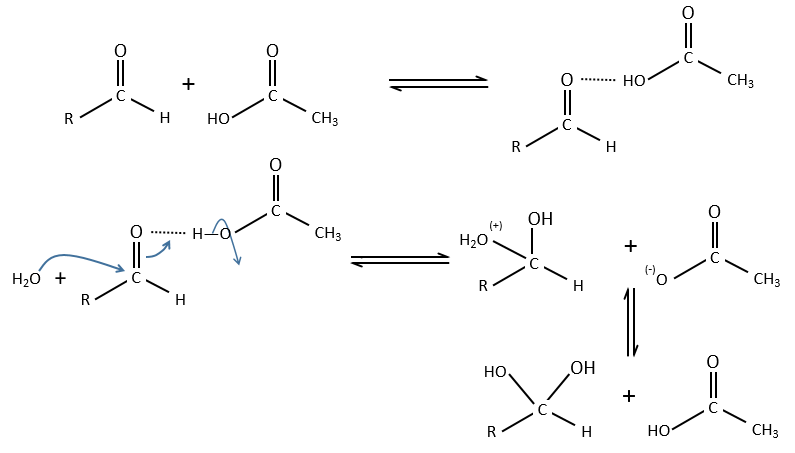
Electrophile catalysis – example of the hydrolysis of an ester
The steric hindrance can make the hydrolysis difficult. In this case we can use anhydric
LiI/pyridine to catalyse the reaction. Li+ is an electrophile that is complexed by the -O-. It acts as an acid of Lewis. The complexation eases the departure of R’ (-CH3 in the present case) which is normally a bad leaving group. K+ or Na+ can also be used.
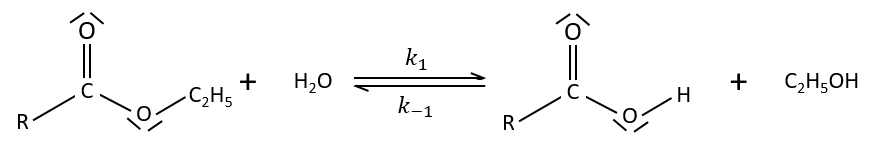
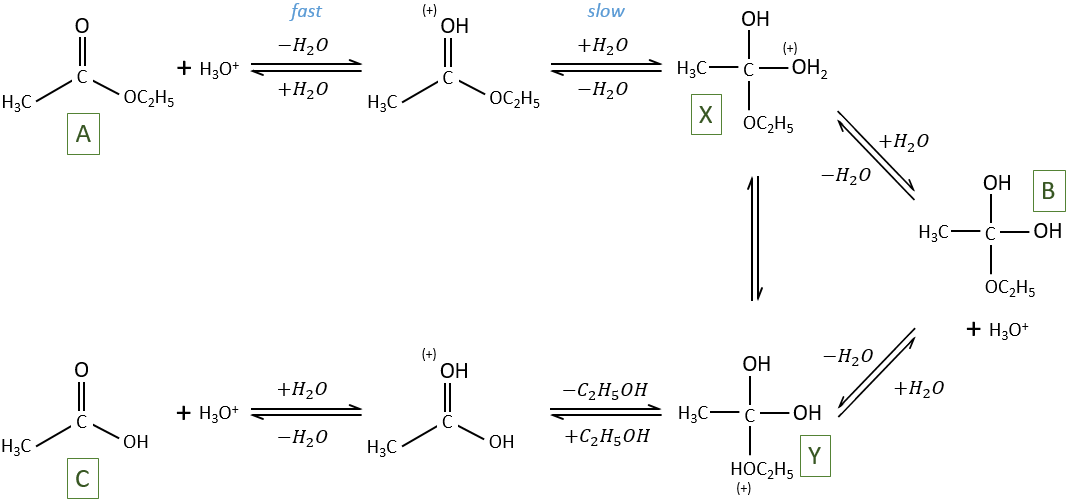
Specific acid catalysis
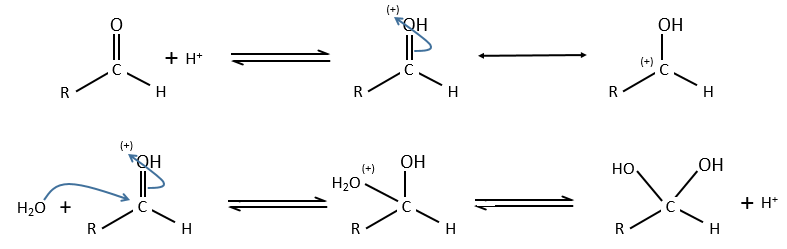
The formation of an acid A from an ester E is very slow in absence of catalyst. Proton can play the role of the catalyst in this reaction. The speed of the reaction will thus depend on the pH of the solution. The source of proton doesn’t matter but H3O+ is the catalyst.
The reaction is reversible but we measure a pseudo order 1 if the acid is diluted: there is almost no product and the reverse reaction is thus negligible.
The concentration of the dissociated acid is calculated by the usual acid-base equation.
If [AH] varies at a constant pH, r is constant and thus kobs doesn’t depend on the concentration of AH. We are in the case of a specific acid catalysis.
The hydrolysis depends thus strongly on the acid-base equilibrium. The mechanism is
The protonation (first reaction) is fast and the nucleophilic attack by H2O (second reaction) is slow. X can turn into Y directly or first into B and then into Y.
General acid catalysis
An undissociated weak acid can also catalyse the reaction. If we have [H3O+] and [HA] constant, then
For a constant ratio [AH]/[A–], kobs varies with the concentration of [AH]. If there is a reaction involving AH, we have to recalculate [H3O+]. If there is no AH in solution, then we are back in the case of a specific acid catalysis.
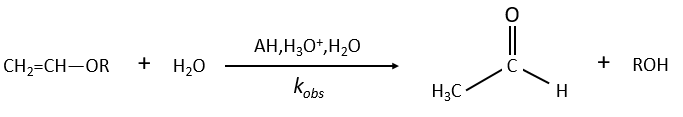
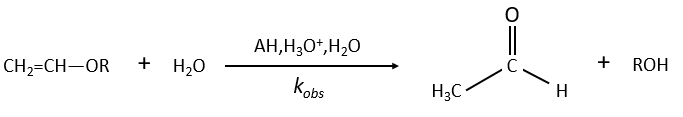
Example of the vinyl ether
The formation of an aldehyde and an alcohol from a vinyl ether (noted V) is catalysed by an acid. In a neutral or basic environment, the vinyl ether is stable (k0»0).
The protons are much more effective than the weak acid and it can be seen from the intensity of the constants of speed. For instance, if R=C2H5, kH= 2.08l mol-1s-1 and kacetic acid= 1.78 10-3 l mol-1s-1.
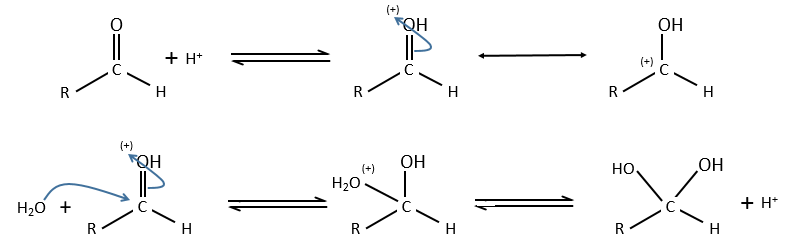
Comparison between specific and general acid catalysis: aldehyde into acetal reaction
In this case there is a difference of mechanism between the general and specific acid catalyses.
Specific:
Protons are directly involved in the first step, so the pH has an influence on the speed of reaction.
General:
A weak acid forms an H bond to enhance the electrophile character of the carbon of the C=O and the transfer of proton is done simultaneously with the nucleophilic attack by the water. No proton are involved so the pH doesn’t change the dynamics. So to determine which catalysis is used, we change the concentration of the weak acid without touching the pH.
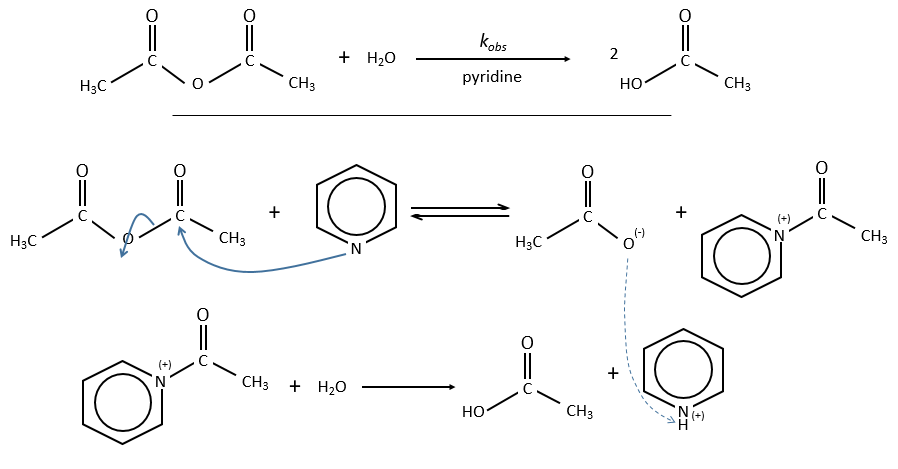
Nucleophilic catalysis
The catalyst interacts with an electrophile centre of the substrate. The process is slower and the intermediate species can be detected by spectroscopy or can be chemically trapped.
Example of the pyridine as catalyst
The acylpyridinium has a half-life time of 0.1s and can thus be detected.
Basic catalysis
Difference between specific and general basic catalyses
Specific:
General:
The NR3-H2O complex is more reactive than water because the lone pair of the oxygen is more nucleophilic.
OH– does not directly intervene in the general basic catalysis, as H3O+ does in the general acid catalysis. Yet the constant of speed depends on the pH because the general and specific catalysis are simultaneous. The general (basic or acid) catalyses are thus an important mechanism at pH’s close to 7, for instance in enzymatic reactions.
Enzymatic catalysis
It can be done several ways
- catalysis by proximity (entropic contribution)
- electrophile and nucleophile catalyses
- general basic catalysis
Catalysis by proximity
We compare here an intramolecular process with an intermolecular process.
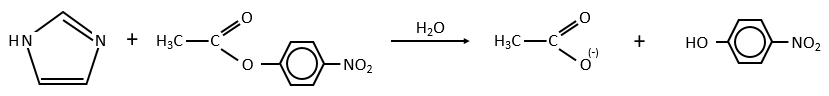
Intermolecular
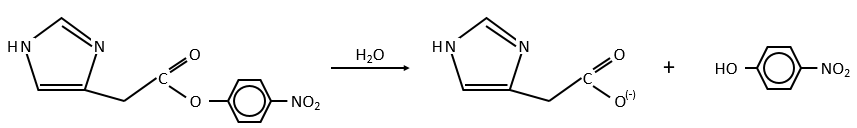
Imidazole is incorporated into many important biological molecules. One of them is the histidine that is present in many proteins and enzymes. The presented reaction is of the second order.
The p-nitrophenol is in equilibrium with its deprotonated form at neutral pH, form that can be detected via spectrometry of absorption.
Intramolecular
The fact that the catalyst was initially bound to the reactant is thus equivalent to the presence of 5.7M of the ester. The fact that the catalyst is already well positioned plays a role and there is also an entropic factor: from one molecule we produce two molecules and there is no need to bind the reactant to the catalyst anymore.
Nucleophilic catalysis
There are nucleophilic groups on enzymes. They can thus act as nucleophile catalysts (see previously).
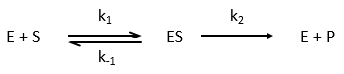
Equation of Michaelis-Menten
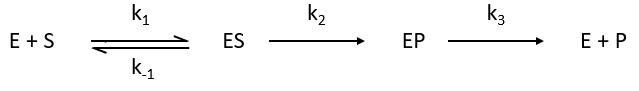
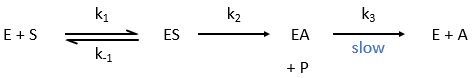
The enzymes are in small quantity in comparison to their substrates. The enzyme forms a complex with its substrate and then acts on it to form the product.
From the section on an equilibrium followed by an irreversible reaction, we know that
In this section, we have seen that there are two limit cases: the irreversible reaction is very fast or the equilibrium has time to be reached. In this case, the equilibrium has time to be established.
The quantity of enzyme is conserved over the time, in its lone form or complexed with the substrate.
We used the hypothesis of stationarity on [ES].
The formation of the product P is given by
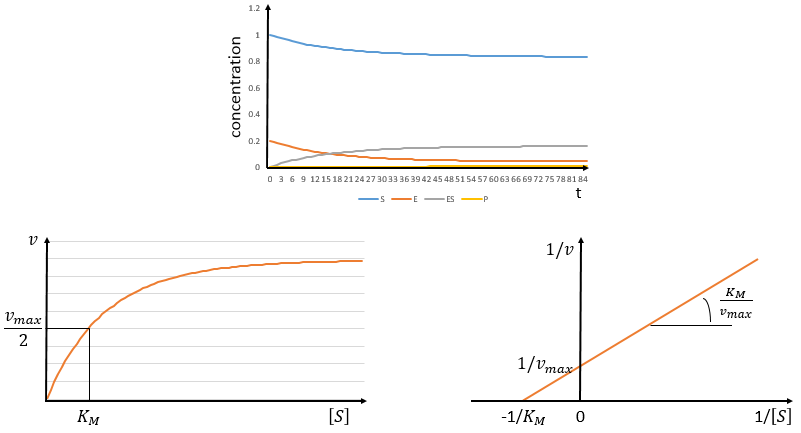
The speed of the reaction depends thus strongly on the concentration of the substrate up to a given point where [S]>>KM. The speed reaches then a plateau at v=k2[E]0. The maximum of the speed is thus reached when all the enzymes are active simultaneously ([ES]=[E]0).
The half of this speed is reached for a concentration [S]=KM.
By plotting 1/v vs 1/[S] (before the plateau), we find a straight line from which we can determine KM and k2.
If we consider one more step in the reaction, the formation of the product when it is still complexed with the enzyme, i.e. before the separation of the species, we will have a correction factor γ to add.
Case of the α-chymotrypsin
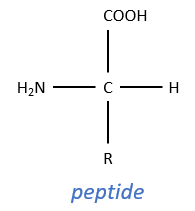
This enzyme is active in the digestive system where it hydrolyses polypeptides. It is active in this basic environment (pH between 8 and 9) and breaks the amide bonds between the peptides.
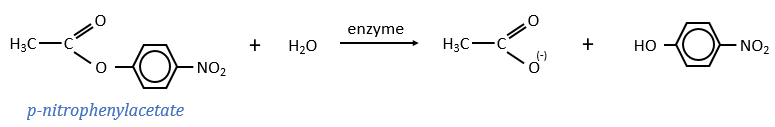
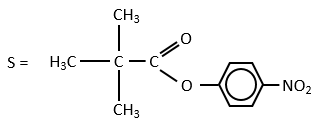
For the experiments, we substitute the polypeptides by an ester, the p-nitrophenylacetate.
The p-nitrophenol is detected due to its yellow colour (405nm) and the acetate is not detected. The observations of the experiment are particular: the enzyme acts very fast at the beginning of the experiment and then the speed reaches a constant level. It is thus the opposite of what we have seen just above. If we look at the small times, the production of phenol is directly proportional to the quantity of enzyme. It seems that it is in this step of the reaction that we have vmax=k2[E]0. There should be a step after the release of the phenol that inhibits the enzyme. This step is the release of the acetate which is much slower than the release of the phenol.
where P=p-nitrophenol, A=phenylacetate and k3<<k2.
As a result, when the phenol is released, the enzyme is not yet available to cleave another substrate. It has first to release the acetate, which is much slower. At the beginning of the reaction, we observe an important and quick increase of [phenol] that is quickly released by the enzyme. Before this moment, all the enzymes can do their job, what explains the large speed of reaction. After, one fraction of the enzymes is still occupied by one fragment of the substrate and cannot react until the acetate is released. The speed of the reaction is thus limited by the third step of the reaction.
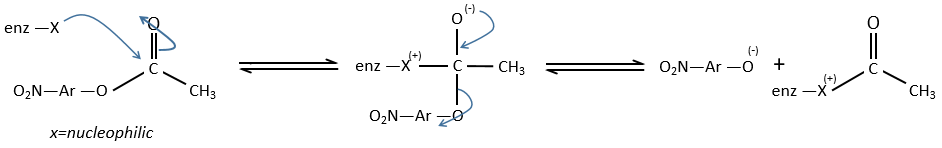
We can assume that there is a nucleophilic site on the enzyme where the substrate binds at the level of the carbonyl.
We can show that the intermediate acylenzyme is correct with the use of a more hindered substrate.
The steric hindrance and the inductive character of the methyl’s slow down the reaction, and thus they increase the life time of the acyled intermediate (~200 minutes). We can isolate the intermediate as crystals and analyse them.
We can use a better nucleophile than water to eliminate the acetate faster.