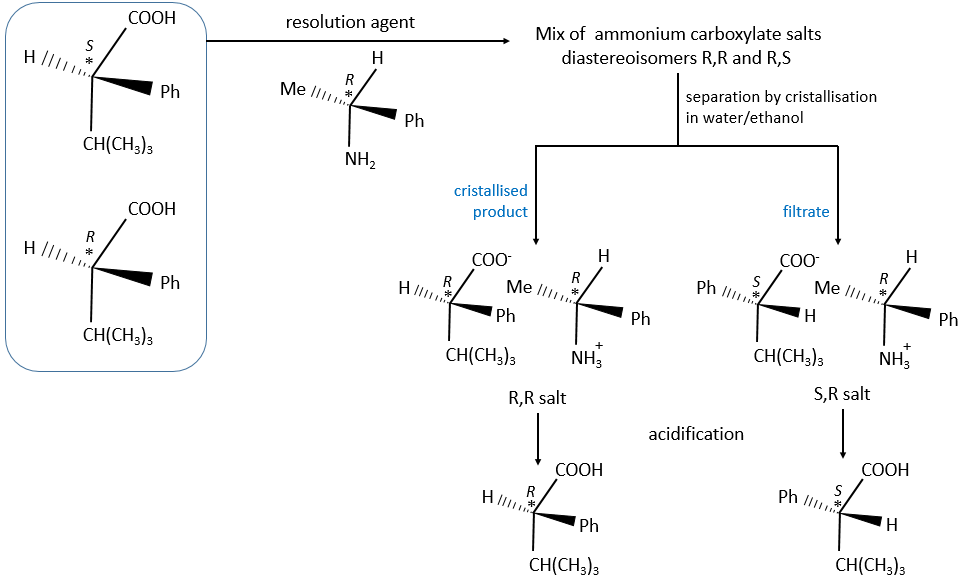
There are two ways to obtain such compounds. The first method is called the resolution in which the reactants we are starting with are either racemic or achiral. We separate the enantiomers after the production of both enantiomers. This method is vastly used in industries even if it doubles the volumes. They try to use the inactive enantiomer in another process. During a diastereoisomeric resolution, the separation of the enantiomers is made by the addition of an optically pure enantiomer called the resolution agent.
The formed salt is a racemic melange of diaseteoisomers that can now be separated.
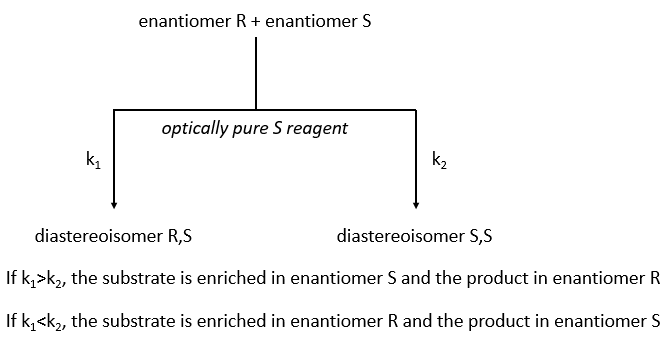
The separation of the enantiomers can also be done via the kinetic splitting that is based on the difference of reaction speed of the enantiomer with a given chiral reactant.
The speed of reaction has to be very different for the two enantiomers. If we put 0.5 equivalent of the chiral reactant, the enantiomer that reacts faster will from the diastereoisomer and the other enantiomer remains intact (ideal case). The conversion ratio is the proportion of the intact enantiomer over the proportion of the formerd diastereoisomer.
The second way is to directly produce the chiral species. This technique evolved over the years and began with the use of a chiral substrate. Around 1980 a chiral auxiliary was used. In this decade, they also used chiral reactants and after 1990 we started to use chiral catalysts. We will describe those techniques soon. First we will talk about the chiral pool.
The chiral pool
The chiral pool refers to natural compound that are optically active and can be produced at large scale (and thus are cheap). It can be aminoacids, peptides, metabolites, etc. We can use those compounds as starting reactants to obtain a chiral final product. The stereocentres already present on the natural compound will control the stereoselectivity of the reactions and lead to one single enantiomer. We talk about a hemisynthesis (because the starting reactant has not be chemically produced).
Amino-acids
At the exception of the glycine, the 20 amino-acids are chiral and available with a good ee (enantiomeric excess). They are bifunctional and levogyre but an enzymatic method can reverse their configuration.
Hydroxy-acids
They wear one or more OH and COOH and can have several stereocentres.
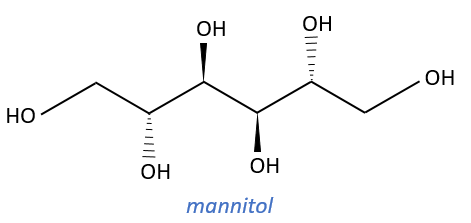
Carbohydrates
They are very cheap but we have to modify them and to protect some groups to use them correctly.
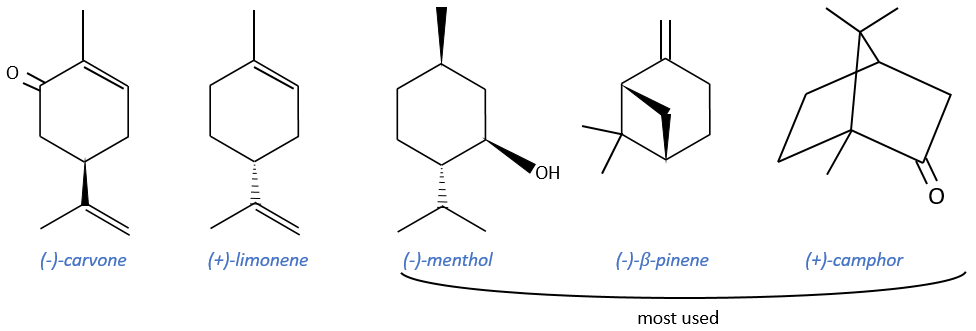
Terpenes
Most of them are used as precursors of agent of resolution or as ligands during asymmetric catalysis but they can also be used as starting reactant of other terpenes. They are cheap and available in large quantities. Their cyclic structure allows a better control of the strereoselectivity. However, they don’t have a lot of functional groups and their optic purity is not always very good.
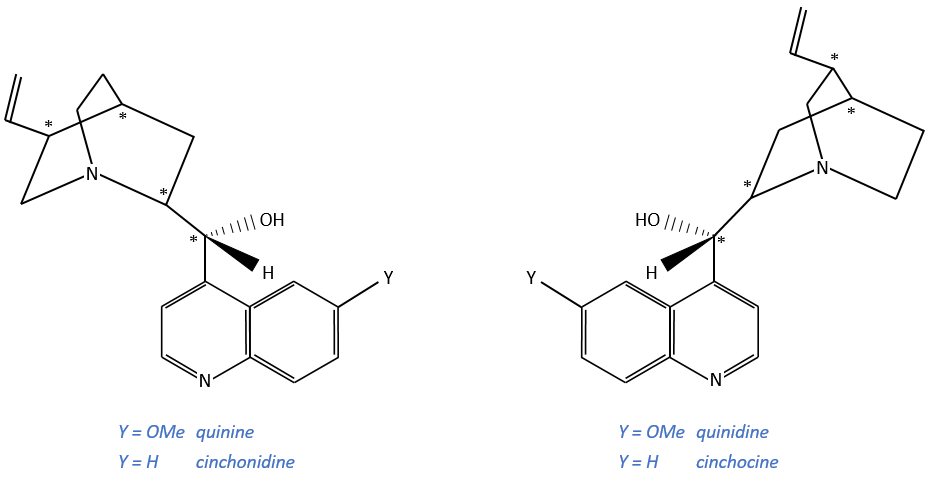
Alcaloïdes
These secondary metabolites possess a nitrogen aromatic and are used as chiral catalysts or agents of resolution.