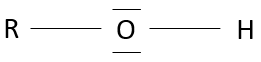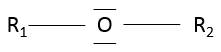Other than alkanes, alkenes, alkynes and halogenoalkanes, there is a large number of functional groups that can be found in organic chemistry. In this first year, we will only take a look on the richness of possibilities that organic chemistry allows through the functional groups a carbohydrate chain can wear, and of some of their properties. We will not yet talk about their reactivity or how to produce them.
Basically, the major atoms in the living, and in organic chemistry, are C, H, O and N. Functional groups are usually made of O or (/and) N, but sometimes other atoms are to be considered.
O based groups
There is a lot of different functional groups containing Oxygen atoms. If both N and O are in a group, I placed this group in the N based group section.
Oxygen is inductive captor and mesomeric donor. Inductive captor means that it attracts electrons of the adjacent carbons because of its electronegativity. Mesomeric donor means that oxygen can share a lone pair with the rest of the molecule, to eventually form resonance structures.
- Alcohols
Alcohols are molecules wearing a OH group on a carbon.
Nomenclature: add –ol preceded by the position of the group. For example ethanol, propan-2-ol, ethan-1,2-diol.
The alcohols can be subdivided in three groups based on the numbers of chains on the carbon wearing the alcohol:
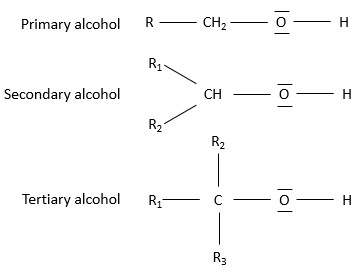
Primary alcohol: One chain is bound to the carbon. The general formula is R-CH2OH.
Secondary alcohol: Two chains are bound to the carbon. The general formula is R1,R2-CHOH.
Tertiary alcohol: Three chains are bound to the carbon. The general formula is R1,R2,R3-COH.
Properties:
Alcohols are weak acids (pKa~16-18). The acidity decreases from primary to tertiary alcohols because the chains share their electrons by inductive effect toward the O. The charge of the conjugate base of the alcohol is thus increased by the presence of chains on the carbon. A larger charge means that the conjugate base is less stable, and will be less probably produced by the dissociation of the proton. Therefore the decrease of acidity. Moreover, the chains increase the size of the molecule, what decreases the solvatation of the ion. That also decreases the acidity of the alcohol.
They are good nucleophiles: The lone pairs of the oxygen can attack electrophile targets.
Alcohols can accept and make hydrogen bonds.
- Ethers
An ether is a group in which an oxygen atom is connected to a second chain: R1-O-R2. Note R1 may be the same than R2, giving a cyclic chain with a heteroatom (=O).
Two nomenclatures are used:
- the names of the chains followed by ether. Examples: dimethylether, ethylmethylether
- the form ‘alkoxyalkane’. The smallest chain is the first noted. Examples: methoxyethane, ethoxypropane
Properties
In general, ethers are volatile and have a low boiling point.
They can be protonated, then form a good leaving group for a SN2. Ethers are often used to protect a group that we don’t want to substitute: form the ether, proceed to the substitution in basic conditions, and remove the ether.
- Ketone
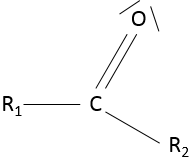
A ketone is a double liaison between a carbon and an oxygen, in the middle of a chain: R1-C(=O)-R2.
Nomenclature: the name of the corresponding alkane with the suffix –one. For example, CH3COCH3: propan-2-one. Note that for this particular compound, a common name is preferred: acetone.
Properties
There is a dipole between the C and O of the ketone. The C is electrophilic while the O is nucleophilic. The oxygen can also accept hydrogen bonds.
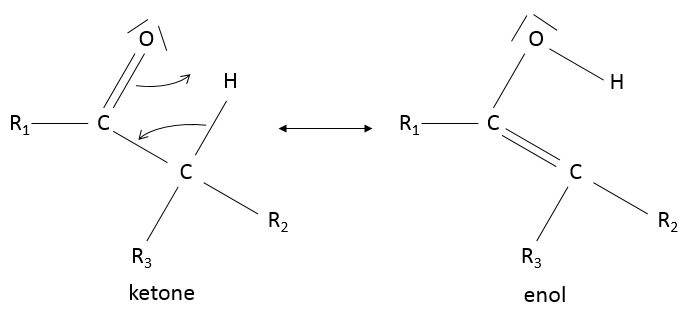
Keto-enol tautomerization: if a hydrogen is on an adjacent carbon of the ketone, a resonance form exist for the ketone, catalysed in acidic of basic conditions
Because of this process, ketones are acidic (pKa~20): the resonance form stabilizes the negative charge of the conjugate base.
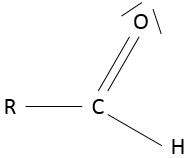
- Aldehyde
An aldehyde is similar to a ketone except that it is at the end of one chain: R-C(=O)-H.
Nomenclature: the name of the corresponding alkane with the suffix –al, for simple alkane chains or –carbaldehyde for more complex molecules. If another functional group is already responsible for the suffix of the name (an alcohol for example), the prefix formyl is used instead. The COH extremity is also called a formyl and the smallest aldehyde, HCOH is called formaldehyde.
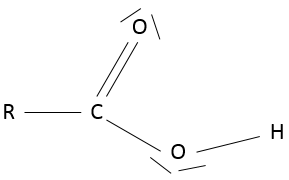
- Carboxylic acid
A carboxylic acid is a ketone bound to an alcohol: R-C(=O)-OH
Nomenclature: the name of the corresponding alkane with the suffix –oic acid. Examples: butanoic acid, propanoic acid. However, there are several common names for some carboxylic acids, derived from what we saw for the previous functional groups: formic acid (HCOOH, from insect stings), acetic acid (CH3COOH), butyric acid (CH3(CH2)2COOH, from butter) and many others.
Properties
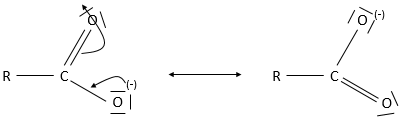
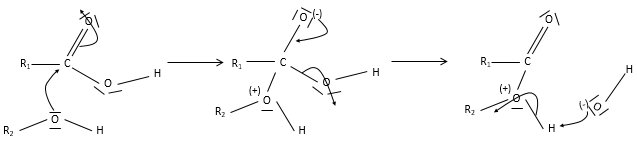
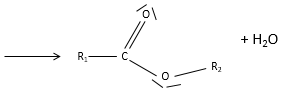
Obviously, carboxylic acids are acid. They are more acid than alcohols and very acidic with regards to the other organic compounds. While alcohols have a pKa~16-18, smaller than the pKa of water, the carboxylic acid is a weak acid, with pKa between 3-5. This acidity comes from the resonance forms of the carboxylate, the conjugate base of the acid:
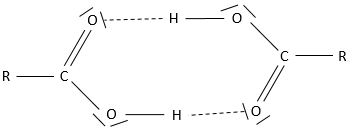
The carboxylic acids are polar and soluble in water (and polar protic solvents), but also in nonpolar solvents: two acids can form a nonpolar dimer by hydrogen bonding.
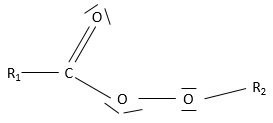
- Esters
An ester is the combination of a carboxylic acid and an alcohol (and it is technically how we can produce esters): R1-C(=O)-O-R2.
Nomenclature: the main chain is the one wearing the carboxylate (R-C(=O)-O-) and is named with the suffix -oate and the rest of the chain is a group. Examples: Ethylethanoate (or ethylacetate), propyloctanoate.
Properties
Esters are polar and can accept hydrogen bonds.
- Peroxides
It is not really a type of group, but more like a modification of existing groups characterised by the bonding of two oxygen atoms.
The peroxide itself is the modification of an ether: R1-O-O-R2.
Hydroperoxide is the peroxide of alcohols: R-O-O-H.
The peracid (or peroxyacid) is the peroxide of carboxylic acids: R-C(=O)-O-O-H.
The perester is the peroxide of esters: R1-C(=O)-O-O-R2.
N based groups
As for the oxygen, the azote is an inductive acceptor and a mesomeric donor.
- Amines
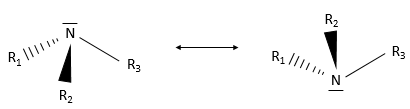
An amine group is an azote atom bound to one or more carbon chains. Primary amines are bound to one chain, secondary amines to two and tertiary amines to three carbon chains. Quaternary amines also exist and wear a positive charge on the azote atom. Secondary and tertiary amines can be found in cycles. So is the piperidine, a very used cyclic amine.
Nomenclature: Whether use the prefix amino- or the suffix –amine. Examples: methylamine, 2-aminohexane.
Properties
Good nucleophiles, depending on the number and the lengths of the bound chains.
Except the quaternary amine, the azote has a lone pair and can oscillate between two conformations.
Amines are bases: the lone pair can accept a proton. The basicity depends on the steric hindrance (and therefore solvatation of the ion) and on the nature of the chains. Alkyl chains are enhancing the basicity (by induction) while aryl chains (an aromatic chain, see next chapter) are decreasing the basicity (by mesomeric donation).
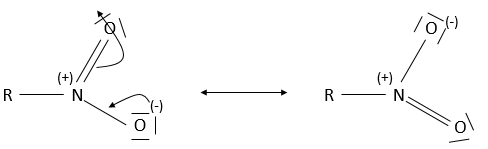
- Nitro group
A nitro group is composed of an azote atom and of two oxygen atoms: R-NO2. It is mainly found on aromatic cycles through electrophilic addition (to be seen next year) and not through nucleophilic substitution.
A positive and a negative charge are respectively on the azote and on one of the two oxygen. In fact, there are two resonance forms for the nitro and the oxygen atoms are equivalent.
Nomenclature: The prefix nitro- is added.
Properties
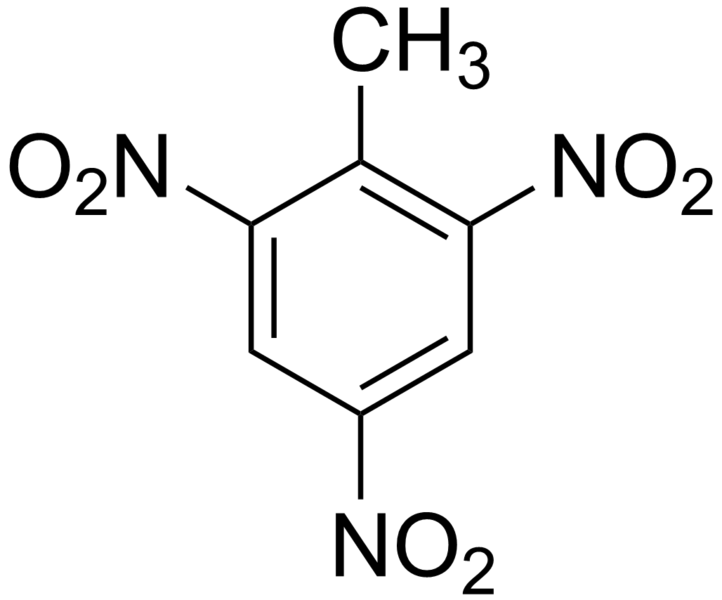
Nitro groups makes compounds explosives. The most known explosive is TNT: Trinitrotoluene.
Don’t heat too much or too quickly a compound containing nitro groups, especially if there is more than one on the substrate.
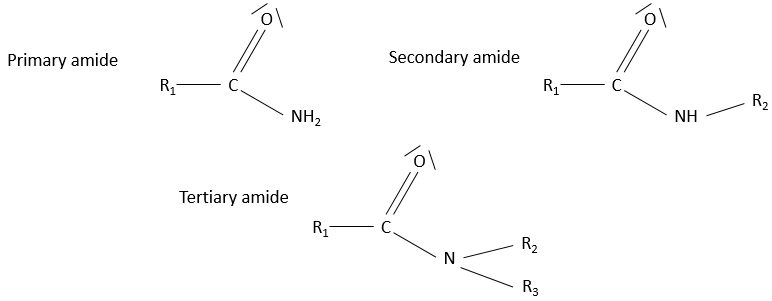
- Amide
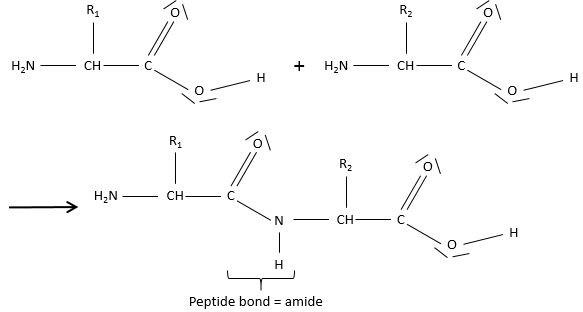
Amides are the equivalent or an ester, except that one of the oxygen is replaced by an azote: R1-C(=O)-NH-R2.
Nomenclature: The suffix amide is simply added at the end of the name of the compound. The equivalent of acetic acid is the acetamide.
Properties
Hydrogen’s bonds possible on the azote in both directions. The solubility of amides in protic solvents is good. The solubility is smaller if the azote is bound to 2 carbon chains (tertiary amide)
Amides are the bone structure of peptides
They have a resonance structure, separating the charges, similar to the keto-enol tautomerization
- Nitrile
Nitriles are functional group composed of C≡N. In inorganic chemistry, this group is instead called cyanide and is very toxic.
Nomenclature: The prefix cyano- is added to the name of the compound.
Properties
They are used to produce carboxylic acids by hydrolysis, or amines by catalytic hydrogenation.
They are also often used to extend a carbon chain.
The carbon is electrophile in nitriles.
Groups based on other atoms
Some other atoms can be encountered in organic chemistry. We will not develop them a lot in this course.
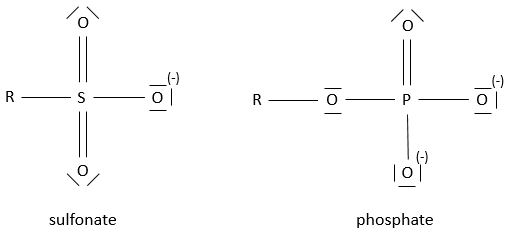
- Sulfonates and phosphates
The prefix sulfo- or phospho- are added to the name of the compound