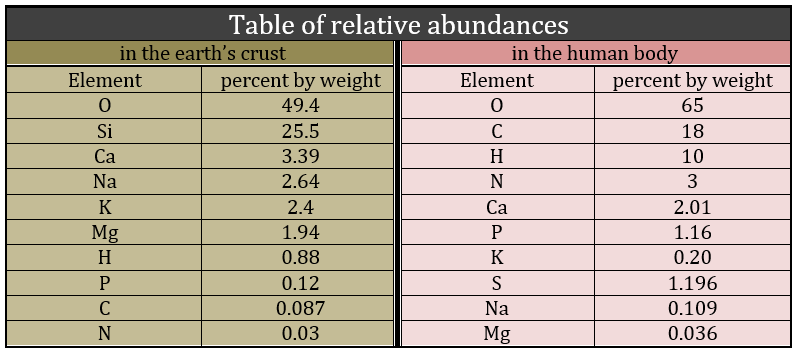
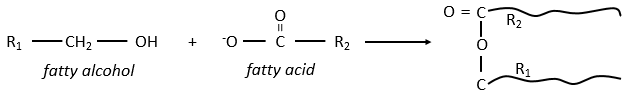Biochemistry is a field of the chemistry related to the living bodies, animal or vegetal. This field is far from the inorganic chemistry and a simple comparison can show it: the repartition of the molecules in the earth’s crust and in a living body are totally different. The first is essentially composed of silica and the second of carbon.
Historically, the first theory about the life was that it is given by God and only by Him. The molecules are inanimate and cannot be turned into a living body except if God breathe life into it. It is called the vitalism theory.
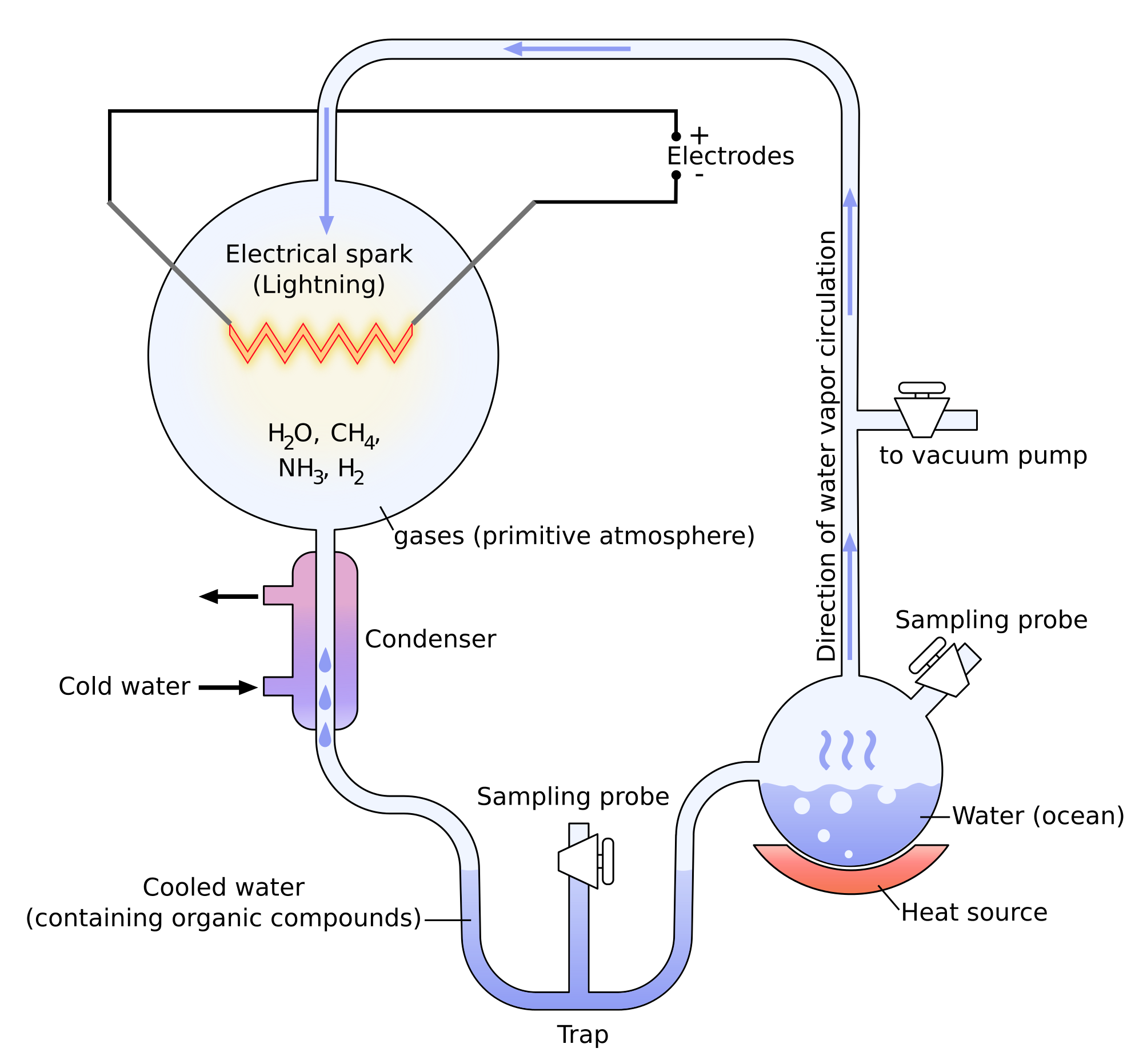
Miller showed through its experiments that it was possible to create molecules belonging to living bodies with simple molecules. The experiments of Miller consist in the modelling of the conditions on Earth before the apparition of the life. There was not a lot of oxygen at this time and the atmosphere was essentially composed of ammoniac, methane, hydrogen and water. The temperature was way higher and there was a lot of lightning. Using these conditions, some molecules that are found in living bodies were spontaneously formed: urea, glycine, alanine, …
Yet there was no trace of proteins and especially of RNA (ribonucleic acids), the molecules capable to write DNA (deoxyribonucleic acids) molecules. It was showed later that some molecules of meteorites allowed the formation of RNA.
Principal characteristics of biochemistry
Limitations
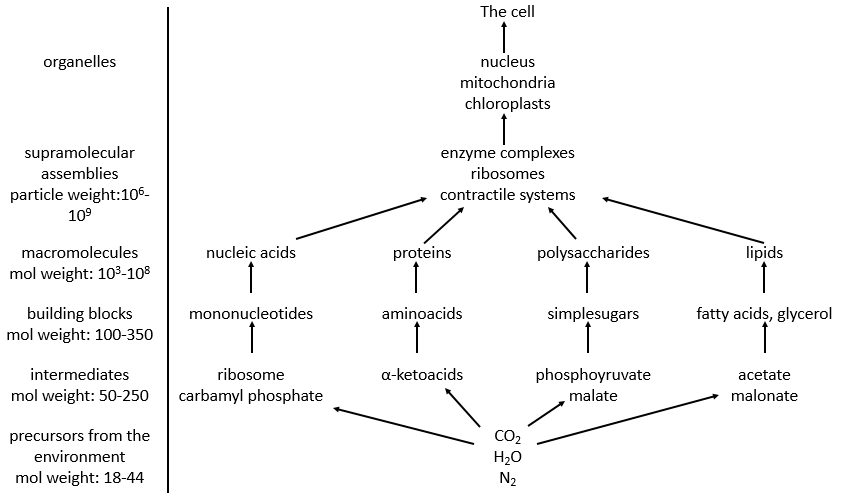
Amongst the good hundreds of atoms in the table of Mendeleev, we only find 16 different liaisons in biochemistry but it allows the formation of an infinity of organic molecules. That is even more surprising that the reactions involved in the biological processes are limited by the biological conditions of existence: there is no way that a reaction that requires a temperature of 100°C takes place in our body. It would damage the surrounding molecules and burn tissues. As any chemical reaction, they must obey the rules of thermodynamic and find the energy required to make the reaction somewhere else.
Specialisation
In a body, the reactions are made in cells and can also be compartmentalised inside the cell. All the cells cannot do all the reactions and some cells are specialised. The specialisation is written in the genetic information and is translated by the presence or the absence of some enzymes. Moreover, in one cell there are not the same kind of reactions in the mitochondria than in the endoplasmic reticulum. It is because the reactions are regulated, positively or negatively, by catalysts.
Enzymes
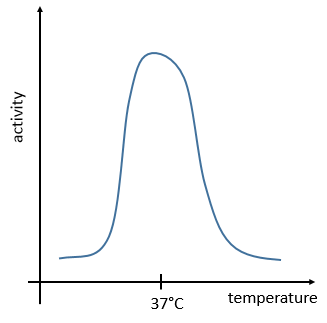
The temperature has to remain low (~37°C) and cannot vary a lot. ∆G=∆H-T∆S has to be negative and close to zero. The speed of reaction would be close to zero without catalysts.
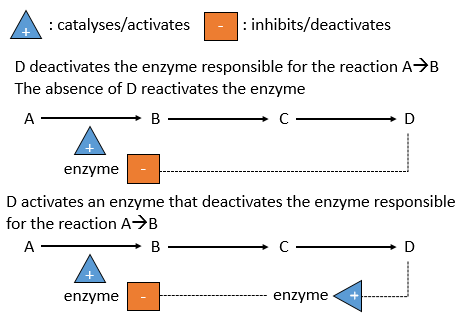
There is a system of regulation in the cells: the activity of the catalysts depends on the requirements of the cell. If the cell is full of one species D, there is no need to accumulate more of it while the resources could be used in a different process. The presence of the species D will influence a catalyst (often an enzyme) that acts on one reaction leading to the formation of D. The presence of D can inhibit an enzyme responsible of the reaction or activate an enzyme inhibiting the reaction. On the contrary, the absence of D can also be detected by this enzyme or another to favour the formation of D.
The management of the energy is a global problem for the body: the processes cannot consume or reject too much heat at once. It could damage the surrounding cells and inhibit most of the enzymes: the temperature of the body is usually in the optimum range of efficiency for the enzymes and if there is a variation of temperature, the activity of the enzymes lowers significantly.
Regulation
Moreover, there are periods during which we consume less energy (when we sleep for instance) and periods of times when the body needs more resources (when doing sport, during a stress, …). These periods do not coincide with the periods during which we produce the energy (when we eat). There must thus be an effective way to store the energy when it is produced and to liberate it when needed. It is done through the formation of ATP (adenosine triphosphate) from ADP (adenosine diphosphate). This reaction is only possible in the mitochondria but the energy is needed everywhere. The energy has thus also to be transported in the different compartments of the cells and in the body.
This course will focus on these problems through the example of the degradation of the glucose. Prior to that, we will make an introduction of the main types of molecules that we find the body.
Molecules of the body
This course will focus on these problems through the example of the degradation of the glucose. Prior to that, we will make an introduction of the main types of molecules that we find the body.
Water
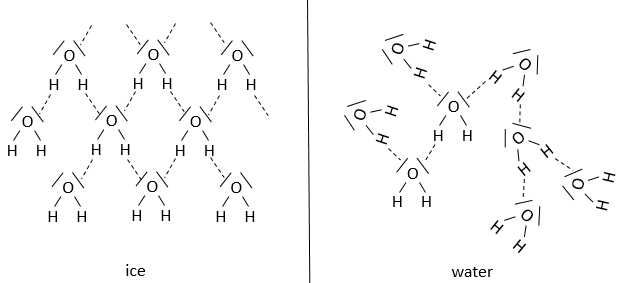
Our body is composed at 60-70% of water. Most of the reactions are thus made in aqueous and polar conditions. The water implies an organisation between the molecules. In the ice, one molecule of water makes 4 H bonds with other H2O molecules.
At the temperature of the body, this number drops to approximatively 3.4. The H bonds break easily and frequently: their energy is ~4.5kcal/mol (against ~ 110kcal/mol for a covalent liaison) and their life time is about 10-9s.
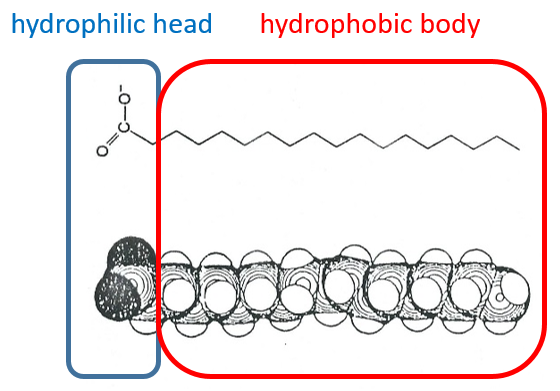
The H bonds bind water molecules together but also water molecule to any electronegative atom (for instance R-OH, R-CO-R, R-COOH, R-NH2…). The groups that form H bonds are called hydrophilic groups. Groups that don’t allow the formation of such bonds are called hydrophobic groups (an aliphatic chain for instance, a phenyl …). The firsts are soluble in water while the seconds are not (they form two separated phases). One molecule that wears hydrophilic and hydrophobic groups is called amphipathic.
The transpiration is a way that the body has find to regulate the temperature: the energy of vaporisation of the water is 540cal/g of water. To compensate the rise of temperature of 1 degree, 2 grams of water are evaporated.
Lipids
Lipids are a group of naturally occurring molecules usually composed of one hydrophilic head and of one or more hydrophobic chains.
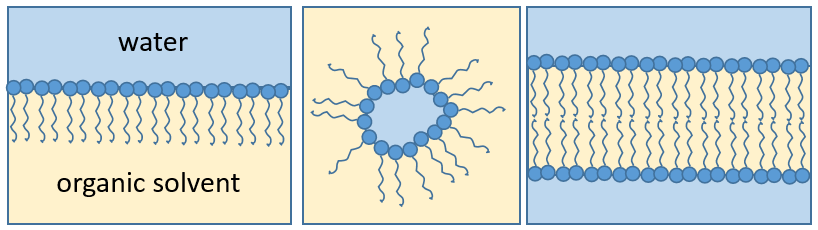
The presence of the polar head does not mean that the whole molecule is soluble in the water. The hydrophobic chains are insoluble in water and aggregate together to minimise the surface of contact with the water molecules. The main biological functions of lipids include storing energy, signalling, and acting as structural components of cell membranes. The membranes they form can either be a monolayer (separating an aqueous and an organic phase) or a bilayer (usually separating two aqueous phases).
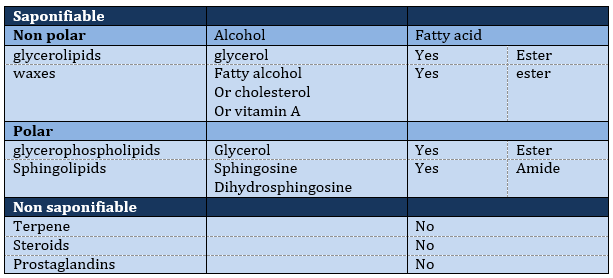
The lipids are sorted in function of the ability to form soap. To do so, the lipid has to contain a fatty acid (one hydrophobic chain finishing with a hydrophilic COOH group).
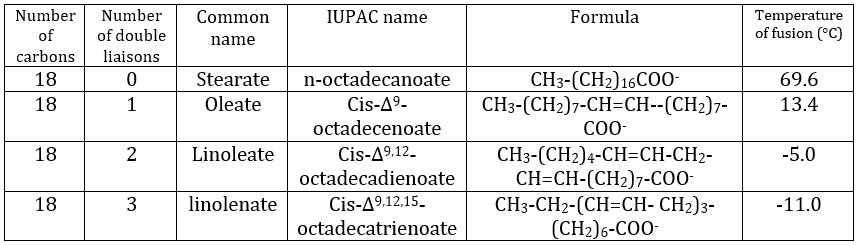
Their nomenclature is ended by ~(an)oate. For instance, CH3(CH2)10COO– (12 carbons) is called dodecanoate. Some lipids wear names coming from the vegetal they come from. For instance CH3(CH2)14COO– is called palmitate because it comes from palm trees. If there is a double liaison in the chain, its position is indicated at the beginning of the name by a ∆x where x is the position of the first carbon with a C=C and the suffix is now ~enoate. For instance, CH3(CH2)CH=CH(CH2)7COO– is called ∆9-hexadecenoate.
The presence of one double liaison hardens the structure of the lipid. One lipid without C=C is said saturated and all the liaisons can rotate. These lipids are very flexible. On the other hand, the C=C fix the conformation locally because the double liaison cannot rotate. As a result, the cohesion between the chains is more complex and the number of interactions of van der Waals decreases (in other words, the chains cannot imbricate as well as saturated chains). A lipid with one double liaison is called unsaturated. If there are several C=C, it is called polyunsaturated. To illustrate the difference one C=C makes, the following table shows a series of lipids with their temperature of fusion.
The unsaturated lipids are found under the form of an oil while the saturated ones under the form of fats. It is possible to hydrogenate the unsaturated lipids to remove the C=C.
Saponifiable lipids
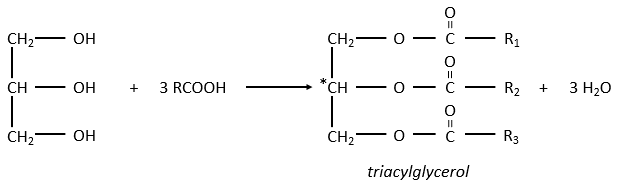
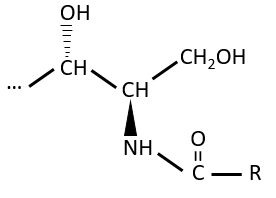
The glycerol is a polyalcohol with 3 –OH groups on a 3 carbons chain. When it reacts with 3 equivalents of RCOO– (a lipid), it forms a triacylglycerol. The carbon noted *C is chiral if R1 and R2 are different. The reaction in the opposite direction is the reaction of saponification:
The reaction in the opposite direction is the reaction of saponification:
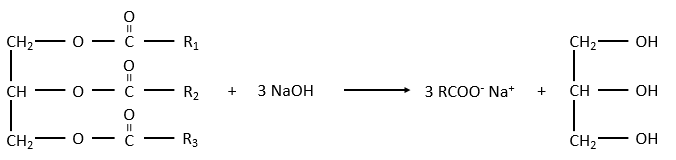
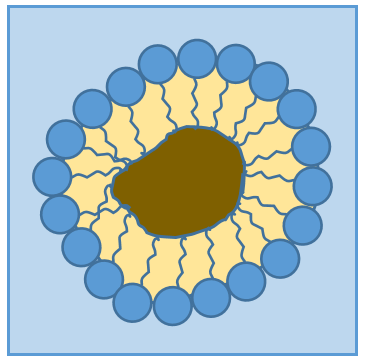
RCOONa is a soap. The principle is quite simple: the soap forms micelles around dirt that are not soluble in water. The hydrophobic chains encircle the dirt and the hydrophilic heads are in contact with the water.
The whole thing is soluble in the water. Lipids are an energetic reservoir stocked under the form of pellets of fat in cells called adipocytes.
Waxes are a combination of a fatty acid with a fatty alcohol.
They are very hydrophobic and are an efficient protection against the humidity (example: the wool of sheep) or to retain the water from evaporating (ex: tropical plants, cactuses). Toothed whales use a mixing of waxes and of triacylglycerol’s, called spermaceti to swim effortless in depths. At 37°C, the mix is an oil but deep the temperature drops and the mix crystallises. Its density increases, what allows the cachalot to float at this deepness.
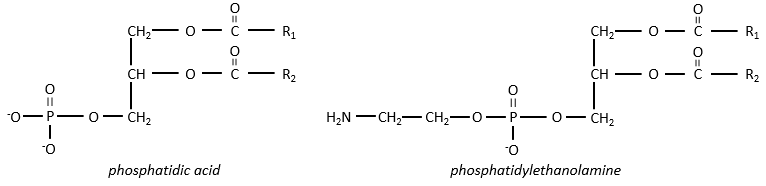
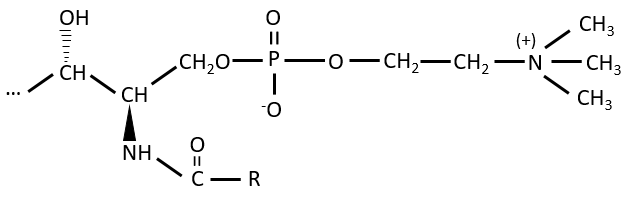
Polar lipids often present a phosphate or a charged amine
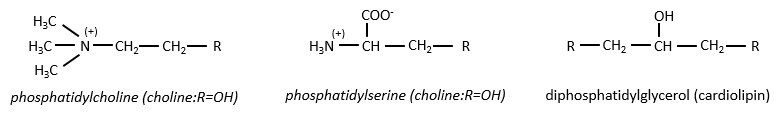
There are a bunch of derivates of the phosphatidic acid. Noting the phosphatidic acid R,
Many of them are constituting the membranes of cell. Oses can also be bound to lipids by via ester liaison and serve, in this case, as an element of signalisation.
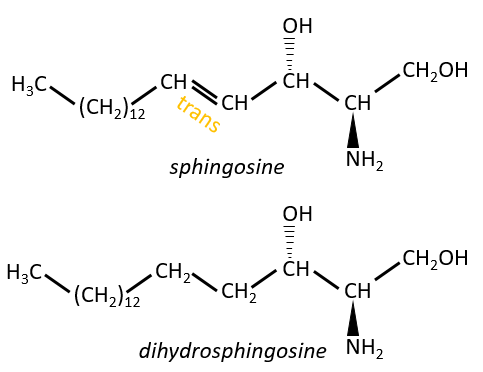
Sphingolipids are derivatives of the sphingosine and of the dihydrosphingosine.
The lipids are bounds via an amide liaison.
Elements giving different properties to the molecule can bind on the alcohol. For instance, a choline can bind there with the help of a phosphodiester liaison.
This molecule is a sphingomyelin and is found in the membrane that surrounds some nerve cell axons. On the ester, an ose can be bound. Those molecules are called neutral glycosphingolipids or cerebrosides because they are often found in the membranes of the brain. Again, the sugar is an element of recognition/signalisation.
The ose can also be acid (for instance a sialic acid) and then the molecule is an acid glycosphingolipid, usually serving as receptor.
Non-saponifiable lipids
Terpenes
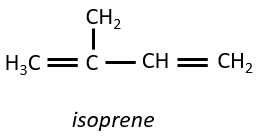
They are small molecules deriving from the isoprene.
The smallest terpenes are the monoterpenes, resulting from the combination of two isoprenes.
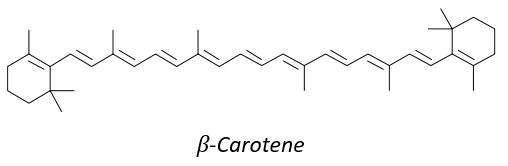
8 equivalents of the isoprene forms the beta-carothene. This molecule of 40 carbons is an antioxidant that protects against free radicals: it is easily excited because the electrons can be delocalised.
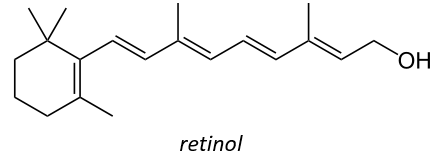
It is also a precursor of the vitamin A. It is a group of unsaturated nutritional organic compounds (found in carrots, amongst others) that includes retinol, retinal, retinoic acid, and several provitamin A carotenoids (most notably beta-carotene).
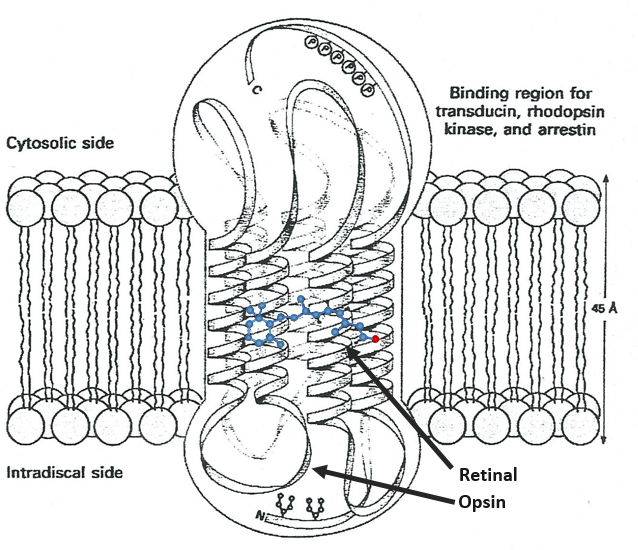
Vitamin A has multiple functions: it is important for growth and development, for the maintenance of the immune system and good vision. Vitamin A is needed by the retina of the eye in the form of retinal, which combines with protein opsin to form rhodopsin, the light-absorbing molecule necessary for both low-light (scotopic vision) and colour vision.
There are two types of captors in the eye: cones and rods. The first detect the colours and the seconds the light. The rods are composed of a series of bilipidic discs with rhodopsin receptors. Between the helixes of opsin is the 11-cis-retinal
All the liaisons except one in the retinal are trans. When a photon hits the retinal, this liaison becomes trans, perturbing the molecule and then the proteinic helixes that transfer the nervous signal. One photon is enough to generate a signal.
In the cones, there are specific modifications in the structure of the protein and the photon has to be in a specific range of wavelength (i.e. one colour) to transfer the signal.
Steroids
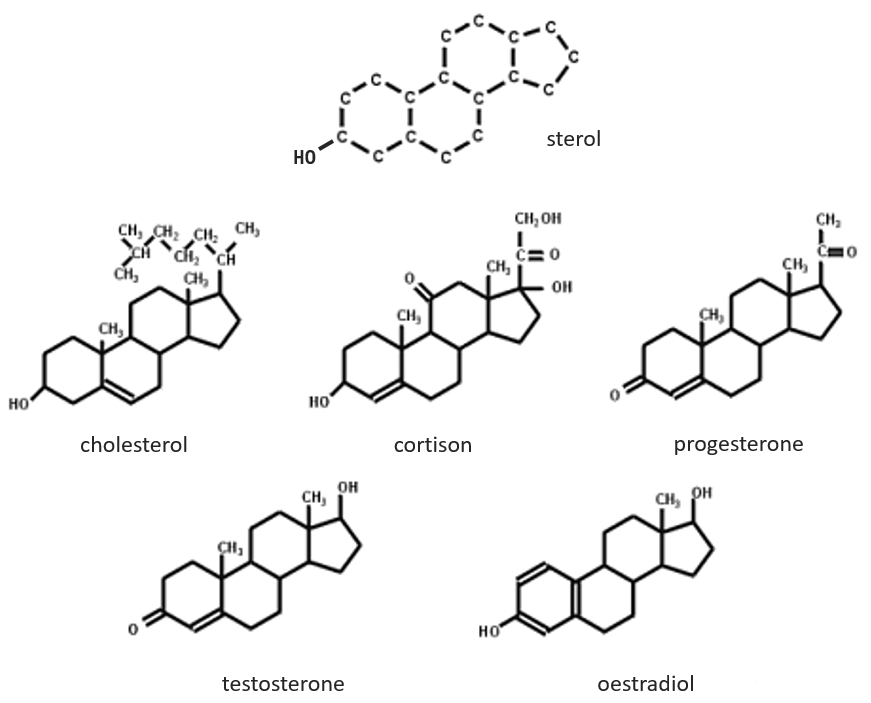
The base of steroids is the sterol, a molecule made of three cycles of 6 C and one of 5 C.
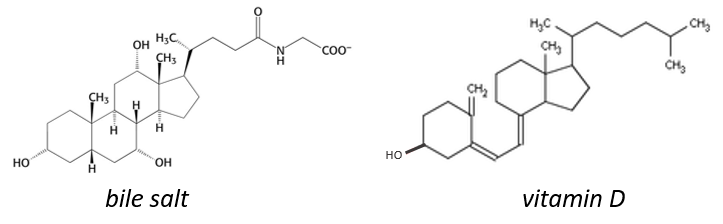
The cholesterol is completely hydrophobic. It is a precursor of several hormones, of the vitamin D and of the bile salt.
We often hear talking about the good and the bad cholesterol. It is actually an incorrect locution. The mode of transportation of the cholesterol is actually the important principle. The cholesterol is transported by lipoproteins (a combination of lipids and proteins) called LDL and HDL (for low and high density lipoproteins). The LDL transports cholesterol in the body from the liver. It is dropped off at some specific receptors to the LDL. If there is too much cholesterol, it will be dropped off anyway. The role of the HDL is to bring back the unused cholesterol back to the liver where it is destroyed. There is thus an equilibrium between the cholesterol that comes from and back to the liver.
Prostaglandins
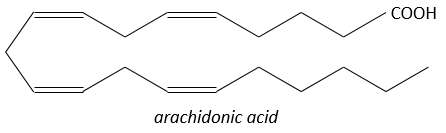
They are hormones acting on a short distance, with a short life time and they are all made from the arachidonate, one lipid of the cellular membrane, by a phospholipase. Prostaglandins activate numerous membrane receptors.
Following a given stimulus, some agents are activated to activate the PLA2 (phospholipase A2). The PLA2 cleaves phospholipids of the membrane to obtain fatty acids. From one fatty acid 4 types of prostaglandins are formed:
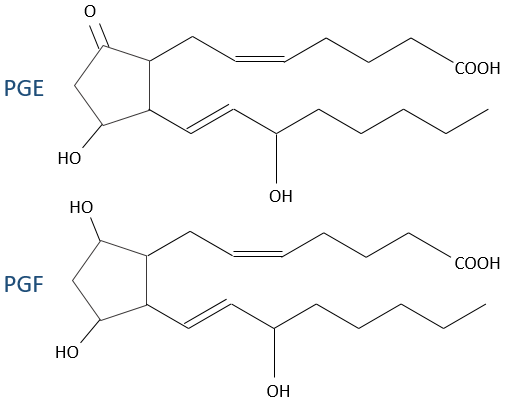
- prostacyclin: they are found in the wall of blood vessels and prevent the coagulation.
- PGE and PGF (stand for prostaglandin E and F): they are responsible of the sleep cycle and from the contractions at childbirth.
- thromboxane: they are implicated in the coagulation process.
- leukotrienes: we find them in the wall of the lungs. Asthmatics have an overproduction of leukotrienes.
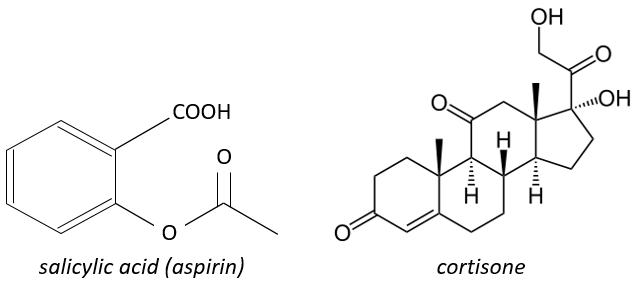
The aspirin blocks the activity/production of the cyclooxygenase which is an enzyme responsible in the formation of the four types of prostaglandins from the arachidonate. The cortisone blocks the PLA2.
Main function of the lipids: constituent of membranes
Membranes of cells separate the inside of the cell from the outside. Both sides are aqueous but the inside of the membrane is hydrophobic. Bilipidic membranes form spontaneously (called liposomes). Membranes are composed of lipids and of proteins with a composition that depends on the cell: basically the proteins allow exchanges and transports from one side of the membrane to the other side while the lipids are the concrete wall of the membrane. Cholesterol places itself in the hydrophobic part of the membrane to consolidate it.