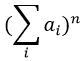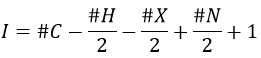Le résultat de la spectrométrie de masse est donné sous la forme d’un histogramme donnant l’abondance des espèces en fonction de leur rapport m/z (on l’écrit aussi M, car les ions sont habituellement monochargés). C’est ce qu’on appelle un spectre de masse. L’abondance est donnée en% du plus grand pic. Peu importe le nombre de fragments, il y aura toujours un pic avec une abondance de 100% et les autres pics auront une abondance plus faible.
Les fragments de faible m/z sont sur la gauche et l’ion parent est, finalement, à l’extrémité droite du spectre. L’ion parent est la molécule qui a été ionisée mais n’a généré aucun fragment. Nous pouvons nous référer à son pic comme le pic parent ou le pic M. Son pic n’est pas toujours présent sur le spectre ou il peut être petit. Il est plus commun d’avoir un pic de parent intense dans le cas d’un bombardement chimique pour lequel un pic à M + 1 est également intense. Des pics avec un M plus grand que le substrat peuvent éventuellement exister à cause des isotopes.
La règle de l’azote :
La règle de l’azote est une règle assez simple qui dit que si la masse de l’ion parent est paire, il y a un nombre pair d’azote dans la molécule. Si la masse est impaire, le nombre d’azote est également impair. Par exemple, il n’y a pas d’azote dans C2H6 et la masse est paire: 2×12 + 6 = 30. L’azote peut porter un atome d’hydrogène en moins (NH3) qu’un carbone (CH4). Si l’on remplace l’un des atomes de carbone par un atome d’azote mais qu’il a aussi une masse impaire, on aura CH3-NH2 avec une masse de 12 + 14 + 5 = 31. Si nous étions en présence de NH2-NH2, la masse est paire puisqu’il y a deux atomes d’azote (M = 32).
De plus, le pic M est généralement faible s’il y a de l’azote dans la molécule.
Les pics isotopiques :
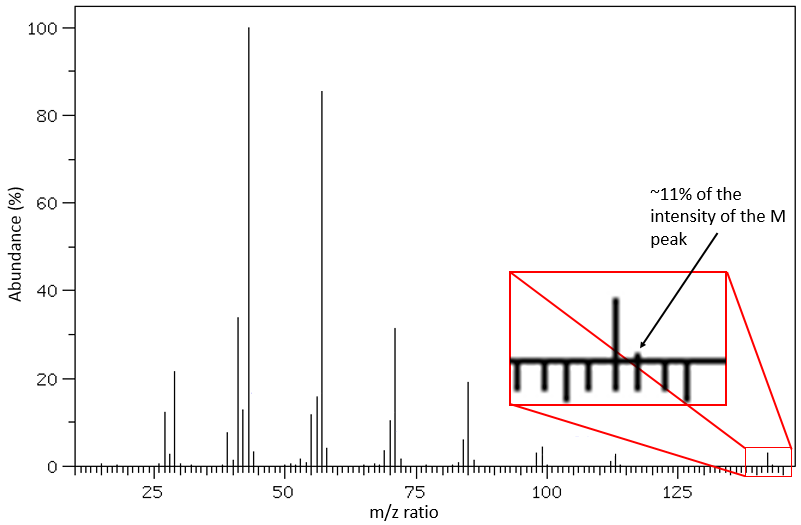
C’est assez rare quand un pic n’est pas directement entouré par d’autres pics d’intensités plus faibles. Ces amas sont dus à la présence d’isotopes dans les fragments. L’abondance de 13C est faible: 1,1% par le carbone dans la chaîne mais des pics à M+1 (M étant le rapport m/z sans isotope) peut être observé si l’intensité du pic principal est grande. Il est également possible que deux 13C soient sur le même fragment, conduisant à un très petit pic à M+2. Nous pouvons estimer la quantité de carbone dans le substrat à partir de l’intensité des pics M+1 et M+2. L’intensité du pic M+1 est égale à 1,1% n de l’intensité du pic M, avec n la quantité de carbone (on ajoute également 0,36% pour chaque atome d’azote N dans la molécule). Cependant, des hétéroatomes tels que O, N et S peuvent également augmenter l’intensité du pic M+1. Cette astuce n’est donc pas absolue.
D’autres atomes peuvent facilement être distingués par l’intensité de leurs pics isotopiques à M+2. Cl et Br ont des isotopes à forte abondance. Fragments possédant un de ces atomes peuvent facilement être repérés sur un spectre de masse. S montre également un pic à M+2 d’approximativement 5% du pic M par atome de S. O montre un très petit pic à M+2 (O.2% par atome d’oxygène). Finalement, Si a des isotopes donnant des pics visibles M+1 (5,1%) et M+2 (3,35%).
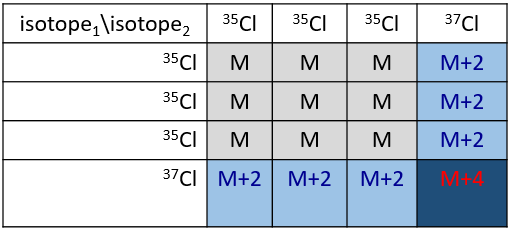
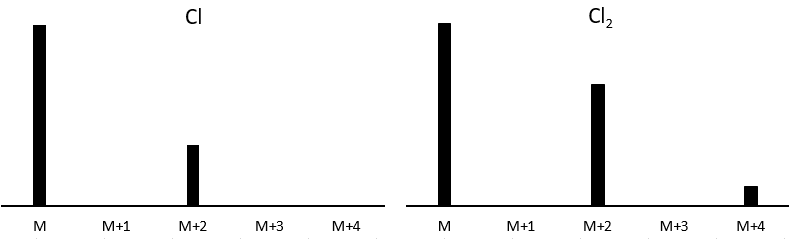
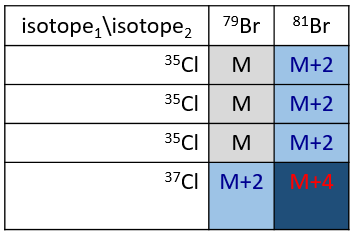
Cl a deux isotopes stables: 35Cl et 37Cl. Leurs abondances sont respectivement de 75% et 25%. Sur le spectre, il en résulte un pic à M+2 avec une intensité correspondant au quart du pic principal. S’il y a deux Cl sur le même fragment, les deux peuvent être 35Cl ou 37Cl ou nous pouvons en avoir un de chaque. Nous aurons donc un pic à m/z = M, M+2 et M+4. L’intensité des pics est toujours liée à l’abondance des isotopes. Pour simplifier, il y a 3 chances sur 4 d’avoir un 35Cl et une chance sur 4 d’avoir un 37Cl pour chacun des Cl. Nous pouvons dessiner le tableau suivant et écrire le pic correspondant dans les cas pour la combinaison correspondante.
Il y a 9/16 des fragments qui auront la masse M, 6/16 la masse M+2 et 1/16 la masse M+4. Le pic le plus intense est toujours le pic avec deux 35Cl. L’intensité du pic avec un 35Cl et un 37Cl à M+2 représente 2/3 de l’intensité de M et le pic à M+4 avec deux 37Cl un peu plus de 10%.
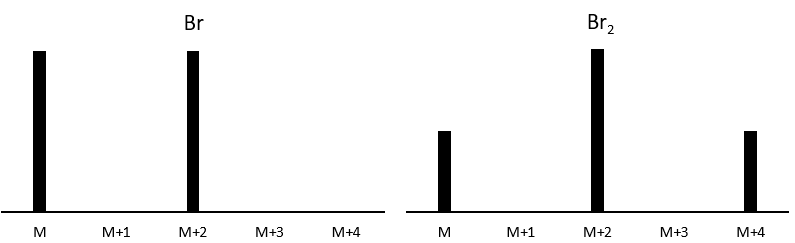
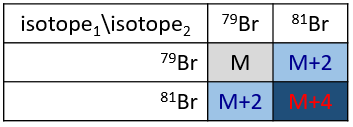
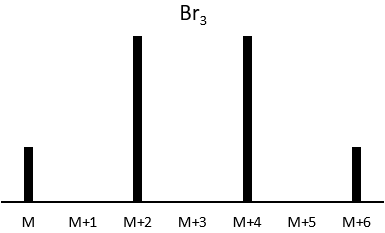
Les abondances des isotopes de Br sont approximativement équivalentes. Si un atome de Br est sur le fragment. Deux pics d’intensités similaires sont séparés par 2 unités de masse. Si deux Br sont sur le fragment, le pic principal est celui qui porte un 79Br et un 81Br (M+2). A M et M+4, les pics ont la moitié de l’intensité de M.
If the fragment has 1 Cl and 1 Br, then we have
The relation between the intensities of isotopic peaks (for one type of isotope) is given by the development of the power of a sum
where ai is the abundance of the isotopes i of the atom and n is the number of atoms of the isotope in the fragment. For the example of the bromine, there are two isotopes with equivalent abundances (50%). If there are three atoms of Br in the fragment, we should observe a repartition similar to
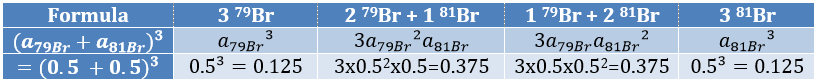
Fragmentation peaks
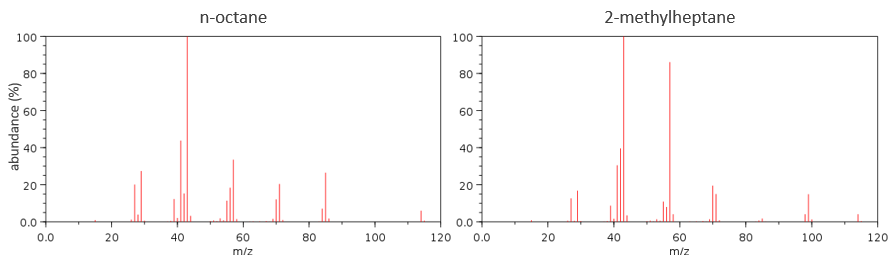
Now that we have analysed the parent peak, we have some advices on the nature on the substrate but we can only guess which molecule it is. A simple example: how would we know if the substrate is the n-octane of the 2-methylheptane?
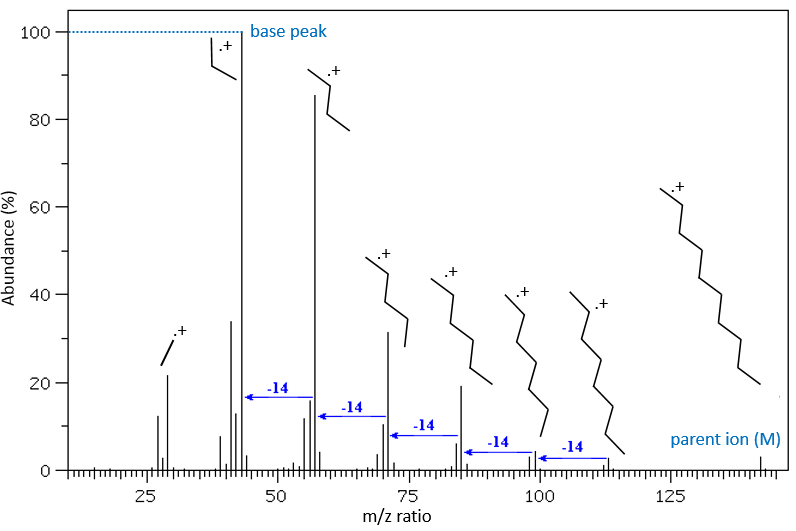
The fragments peaks can help us to find out. To talk about the fragments, we refer to peaks by their m/z ratio. For instance, we can say that the parent ion is at M=m/z=114 and that the base peak is at m/z=43 for both species, and that they have peaks in common (m/z=29, 43, 57, 71 and 114). The intensities of those peaks are not identical and the n-octane shows a peak at m/z=86 and no peak at m/z=99. The fragmentation was thus different because of the structure of the molecule, the composition being identical.
Imagine now that we only knew that the two molecules are alkanes. From the parents ion we can determine that both of them are a form of octane. The interest of the fragmentation peaks will be to give us advices on the structure of the alkanes.
It can be useful to draw the molecules of our guess and cut them into fragments. Virtually, the molecule can break anywhere. A method is to draw the semi-developed representation of the molecule.
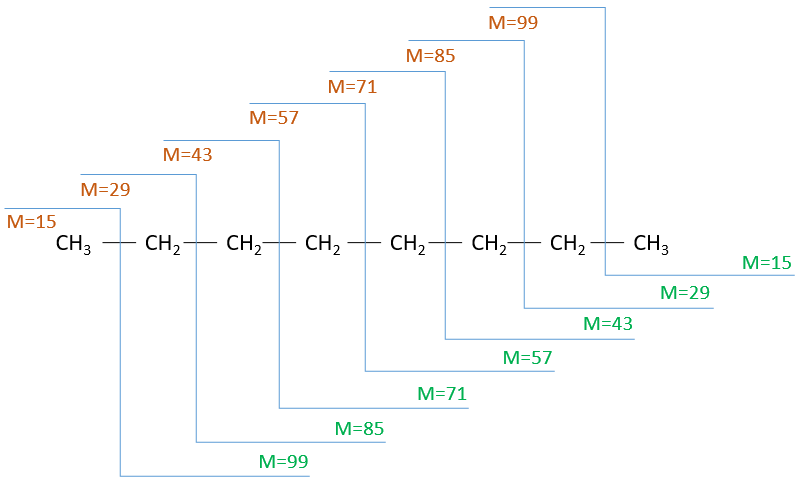
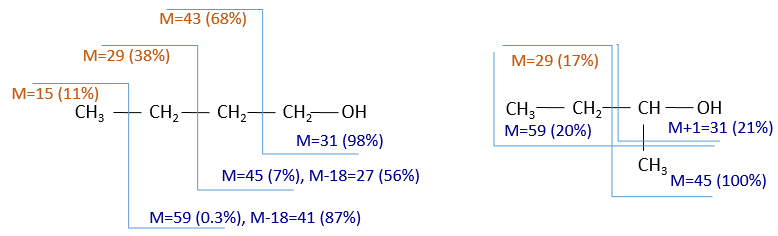
To represent the fragmentation at one liaison, we draw a line perpendicular to the liaison and indicate the masses of the fragments at the left and right of the liaison (respectively in orange and green on the figure above). The Z shape drawn on the figure above is more convenient than a simple line in the case of more complex molecules. For instance, if the n-octane is cleaved after CH3-CH2, the fragment of the left has a mass of 29 (units of mass) and the second fragment, i.e. the rest of the molecule, has a mass of 85. These fragments can be found on the spectrum of the n-octane and have approximatively the same abundance. Unfortunately, the abundances of two complementary fragments are not linked. Remember that when we obtain a fragment, only one side of the molecule remains charged and is detected by the MS, the second fragment being neutral and thus not detected.
One can see that the fragmentation at the level of the –CH3 does not appear on the spectrum. It is because the probability of fragmentation for a given liaison is related to the strength of the liaison, to the possibility of a low energy transition and to the stability of the generated fragments. In general, the intensity of the peaks are not identical and it is common to observe one or two very intense peaks amongst the fragmentation peaks. Some general rules may help us to predict the nature of the most intense peaks:
- the intensity of the M peak is the highest for linear chains and decreases with the degree of ramification. One can see it on the previous figure.
- the intensity of the M peak decreases with the mass of the substrate (for similar structures). The fat esters are an exception.
- aromatic compounds usually show intense M peaks. Double liaisons, cycles and aromatic cycles stabilise the molecular ion, leading to an intense M peak.
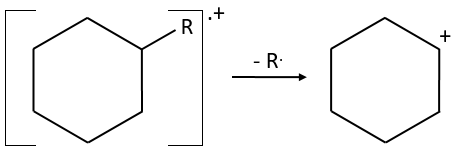
- the fragmentation is favoured at substituted carbons because it leads to carbocations that are more stable. The series for the stability of carbocations is
The rule is thus similar to the one for the stability of carbocations for SN1. The biggest substituents are usually removed more easily and there is usually no peak at M-15 because CH3+ is not stable. If a peak is present at M-15, it definitively means that there is a ramification in the molecule.
- Double liaisons favour the allylic cleavage to stabilise the carbocation.
- liaisons nearby heteroatoms are frequently broken because of the possible stabilisation of the charge by resonance.
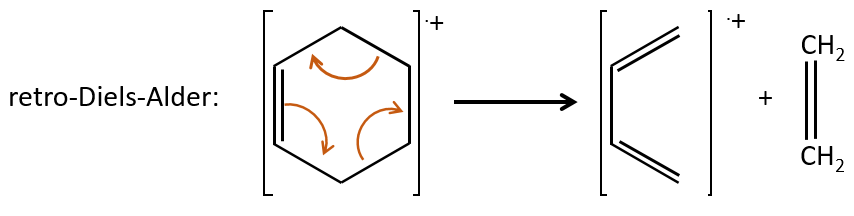
- saturated cycles usually lose alkyl chains and keep the positive charge. Unsaturated cycles are subject to a retro-Diels-Alder reaction.
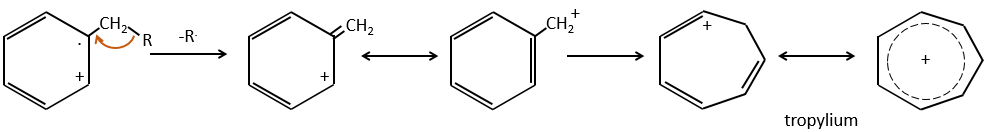
- aromatic cycles usually keep a methyl group and fragment in beta of the cycle to stabilise the positive charge and give the tropylium ion by rearrangement.
- the cleavage is favoured when small neutral molecules can be removed, such as water, H2S, NH3, HCN, CO, etc.
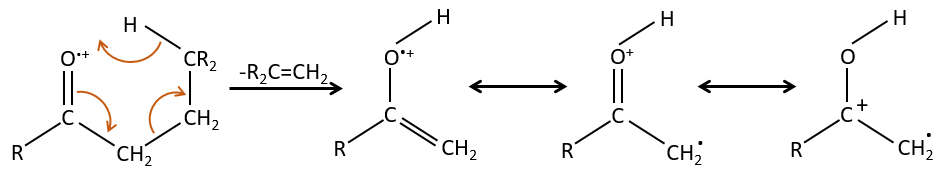
- rearrangement of Mc Lafferty if a hydrogen is in γ of carbonyl. Some fragments cannot be explained be simple cleavages of liaisons from the molecular ion. Such fragments can be the result of an intramolecular rearrangement. The rearrangements often involve the migration of a hydrogen atom on a heteroatom. For instance, a hydrogen in γ of carbonyl can migrate on the O.+. The resulting cleavage is done on the liaison in αβ of carbonyl. It is called a rearrangement of Mc Lafferty.
Typical fragments
There are two ways to look at the fragmentation peaks, and they are used together. The first way is simply to determine what they can be from their m/z, as we have done with the parent peak. There are tables of possible ions corresponding to each m/z ratio.
Here comes a list of some characteristic ionic fragments (that are thus detected on a spectrum):
M between 19 and 25: unusual except if there is a O or F.
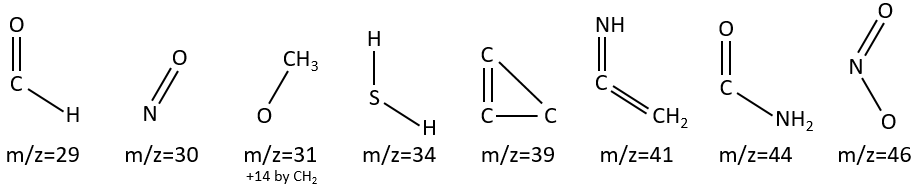
M=29: presence of an aldehyde.
M=30 (or 46): presence of a nitro.
M=31+n14: presence of an etheric oxygen.
M= 34: presence of a primary -SH.
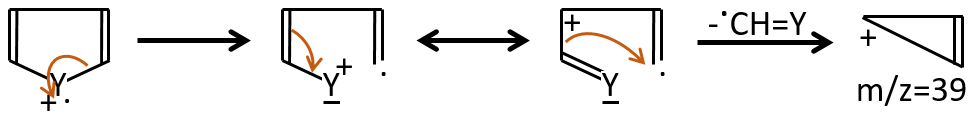
M=39: presence of a heteroaromatic atom in a cycle of 5 atoms. Several complex cleavages can occur in heteroaromatics, giving respectively the ions C3H3+ (m/z=39), HCY+ or CH2Y+.
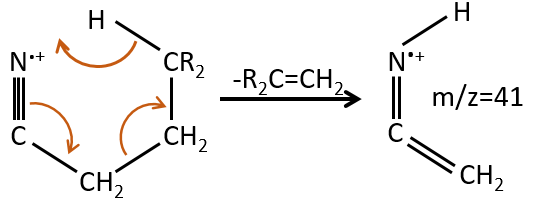
M=41: presence of a nitrile (if Mc Lafferty).
M=44: presence of a primary amide (if Mc Lafferty).
M=46 (or 30): presence of a nitro.
M=47: presence of a sulfur.
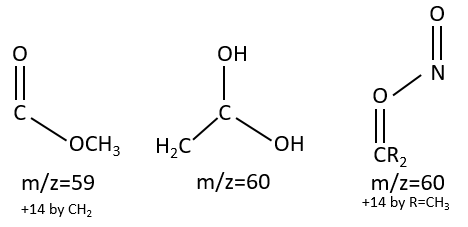
M=59+n14 (clusters): presence of an ester.
M=60: presence of an acid (if Mc Lafferty).
M=60+n14: presence of an aliphatic nitrite.
M=61: primary or secondary -SH.
M=77: this peak is intense and is characteristic of aromatic cycles. It represents the fragment C6H5+. This peak is often in a cluster of peaks (C6H5-6-7) and is accompanied by a peak at M=91 for the toluene/tropylium ion.
M=97: presence of a nitrile (if Mc Lafferty) with a long chain able to form a cycle of 6 carbons.
The other way is to determine which particle has been lost from the parent ion (or from another fragment) to obtain the fragment we are looking at. On the spectrum of the linear alkane that we have seen at the beginning of the section, there is a regular Δm/z=14 between fragments, corresponding each time to the removal of one CH2 fragment of the chain.
It does not mean that the parent ion broke into one fragment by losing one CH2, then that that fragment loses CH2 afterwards, that itself loses CH2 etc., but that the parent ion can break at several places of the chain with a given probability proportional to the intensity of the peak. If the chain is not linear, the distances between the fragments are not so regular. It is why we can say that the spectrum of the left is the one of the n-octane and the second spectrum is the one of the 2-methylheptane.
Here comes a list of some characteristic fragments (neutral or radicals) that can be removed from the molecule:
M-15: -CH3
M-18: -H2O
M-30: -NO
M-31: -OCH3
M-33: -SH
M-45: -COOH
M-46: -NO2
The presence of halogens, spotted by the presence of intense peaks at M+2/M+4 can also be confirmed by the presence of a peak at M-X with X the mass of the halogen.
Confirmation of the composition and structure
Once we determined enough fragments (present on the spectrum and lost), we can try to puzzle them together. At this point, we should have a clear idea of the formula of the molecule. With the formula, we can determine the degree of unsaturation of the molecule. It is given by
For instance, C7H7NO has a degree of unsaturation I=5. The atoms that are bivalent, such as O, are not counted. The atoms with the same valence than C, H, N and O have the same value of unsaturation. S has a value of 0, P has a value of +1/2, etc.
One unit of I represents 1 double liaison or one cycle. Triple liaisons are counted as two double liaisons. A phenyl has thus a value of 4 (3 C=C and 1 cycle).
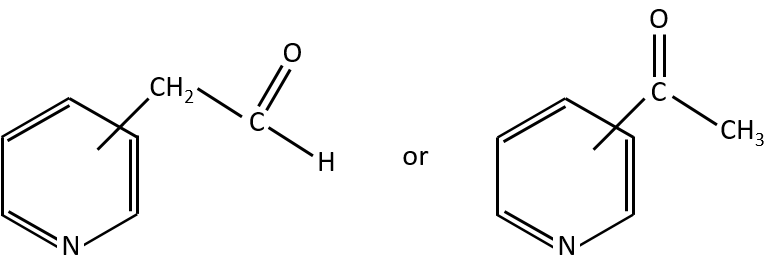
Y can be a carbon but also a heteroatom. The molecule corresponding to the formula C7H7NO can thus be
In combination with the mass spectrum, we should be able to determine which molecule it is.
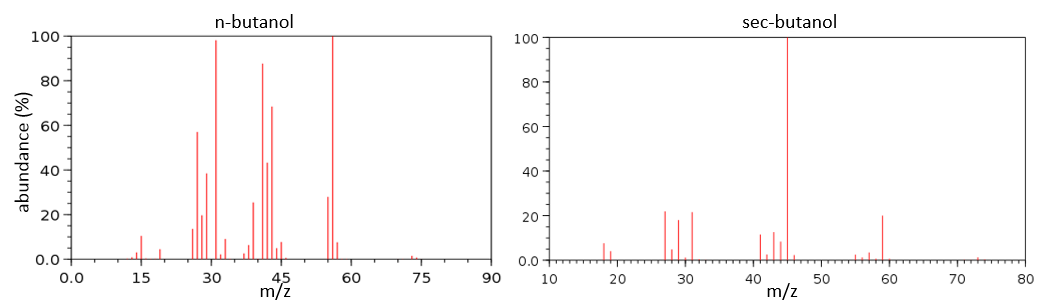
To check our guess, it can be useful to draw the molecule and cut it into its fragments. For instance, the n-butanol and the sec-butanol have two very different mass spectra. Some peaks are on the same location but their intensities are different, referring to the probability of cleavage at a given liaison. It is not necessary to explain all the peaks.
We gave here a strong resume of the mass spectrometry technique and of the analysis of a spectrum. The manual analysis of a spectrum is something that you learn by doing it again and again and that may require some guessing (sometimes some small peaks are important to determine the structure of the molecule and some intense peaks are clueless, like the peak at 56 of the n-butanol). Each group (alcohol, ether, ester, etc) have particular fragments or rearrangements and it is not the point of this section to describe all of them. It will eventually be the subject of a further section (it represents more than 20 pages in my book of reference (Spectrometric identification of organic compounds, Silverstein, Basler, Morill) and the tables for the fragments are 50 pages long, ≈150 fragments per page.
Despite its huge power, there are often several structures than can correspond to one mass spectrum. Usually, the mass spectrometry is used in combination with IR/UV spectrometry and/or NMR. Combined together, we can determine not only the structure of the molecule, but also the exact conformation. We will discuss those techniques in the following sections.