New treatment of inflammatory bowel diseases by autonomic nervous system remodeling
Epidemiology
- Higher incidence (9 – 20/100,000 person years) and prevalence (156 – 291/100,000 people) in populations of North American and Northern European descent (Lancet 2012;380:1606)
- Incidence increased in industrialized countries and urban versus rural locations, suggestive of environmental triggers, such as improved sanitation, reduced exposure to childhood enteric infections and mucosal immune system maturation (Lancet 2012;380:1606)
- Bimodal age distribution with peaks at 15 – 30 years and 50 – 70 years (Lancet 2012;380:1606)
- Family history of inflammatory bowel disease, particularly that of a first degree relative (5.7 – 15.5%) and Ashkenazi Jewish descent (3 – 5x) show higher risk of disease development (Lancet 2012;380:1606)
- Gastrointestinal infections with Salmonella spp, Shigella spp and Campylobacter spp have twice the risk of developing ulcerative colitis postinfection (Lancet 2012;380:1606)
- M = F
- Former cigarette smoking is strong risk factor (Lancet 2017;389:1756)
Sites
- Almost always involves the rectum
- Continuous pattern of involvement proximally to include up to the entire colon (pancolitis)
- Rectal sparing can be seen, particularly after treatment (Lancet 2012;380:1606, Histopathology 2014;64:317, Am J Clin Pathol 2004;122:94)
- Patch of inflammation in the cecum, often involving the periappendiceal mucosa (cecal patch), can be present (Lancet 2012;380:1606, Histopathology 2014;64:317)
- Approximately 20% of patients will have inflammation in the terminal ileum (backwash ileitis)
- Typically present in patients with pancolitis (Am J Surg Pathol 2005;29:1472)
- Focally enhanced gastritis can be seen in approximately 20% of pediatric patients (Pathology 2017;49:808)
- Extraintestinal manifestations:
- Peripheral arthritis, seronegative
- Ankylosing spondylitis or sacroiliitis
- Erythema nodosum
- Pyoderma granulosum
- Primary sclerosing cholangitis (PSC)
Pathophysiology
- Not fully known but appears to be a complex multifactorial process involving an overwhelming T helper type 2-like immune response, leading to mucosal injury in response to gut microbial dysbiosis in genetically predisposed patients
- Proposed mechanisms include:
- Damage to the colonic epithelial barrier due to dysregulation of epithelial tight junctions, which provide a physical barrier between the immune cells and the luminal microbes, leads to increased permeability (Lancet 2012;380:1606)
- Colonic epithelium upregulation of antimicrobial peptides, known as beta defensins (Lancet 2012;380:1606)
- Disruption in the homeostatic balance of the mucosal immunity and the enteric nonpathogenic bacteria, resulting in the patient’s aberrant immune response to the enteric commensal bacteria (Lancet 2012;380:1606, Front Microbiol 2018;9:2247)
- Increased number of colonic epithelium activated and mature dendritic cells with increased stimulatory capacity (Lancet 2012;380:1606)
- Increased expression of TLR4 by lamina propria cells and TLR4 polymorphism, which can alter susceptibility to enteric infections and tolerance to commensal bacteria (Lancet 2012;380:1606)
- Disruption in the homeostatic balance between regulatory and effector T cells, leading to a nonclassic natural killer T cell production of IL5 and IL13, which have cytotoxic effects on epithelial cells, mediating an atypical Th2 response
- IL13 can induce a positive feedback system on the natural killer T cells, leading to increased tissue injury (Lancet 2012;380:1606)
- Increase in proinflammatory cytokines, chemoattractants such as CXCL8 and adhesion molecules such as MadCAM1 recruit increased leukocytes to the colonic mucosa (Lancet 2012;380:1606)
- Other genetic risk loci include IL23 and IL10, JAK2 kinase pathway genes, hepatocyte nuclear factor 4α, CDH1 and laminin β1 (Lancet 2012;380:1606)
Etiology :
I propose a mechanism that involves the limbic system and that continuously maintains a state of stress equivalent to a situation of major stress (trauma) and therefore causes all the signs and symptoms of the disease. How does that happen?
Imagine the situation of this gazelle:
Imagine the situation of this gazelle:

Its limbic system will use all means to save its life, by starting it will increase blood circulation in the muscles by dilating the arteries of the muscles (vasodilation), it will increase blood pressure to increase the pressure of perfusion, it will increase heart rate to increase blood flow per minute. This system will trigger other reactions at the level of other organs for example, in the liver and fat tissue it will cause an increase in the release of carbohydrates and fatty acids to bring the necessary energy to the muscles. It will give to this gazelle a deeper field of vision as well as many others possibilities with other systems (see physiopathology of stress) but this system will go further it will reduce the circulation in the internal organs to offer the muscles the blood flow necessary for survival (its survival is at stake the intestines and other organs can wait).
It will also stop the movement of the intestine and the evacuation of the urinary system, because this is not the time to go to the bathroom!So no defecation and no urination. At the same time for the same reason the appetite is inhibited But while this gazelle is running it will see a lot of things it will see flowers, plants, streams, trees, the sun, as well as other animals…… in short it will see its usual environment in complete.Probably consciously it will record only the traumatic part of what happens to it, because it is concerned about its survival, but subconsciously all these elements will be recorded. As a result, it will consciously record a small portion of the information and a large portion of the information will be recorded subconsciously. If this gazelle succeeds in saving its life, it cannot help but record those moments of stress in his memories.
After this event this gazelle is condemned to live with the stress that is recorded and this state of stress will have a continuous effect because the environment of this gazelle’s life is filled with elements which unconsciously remind it of the stressful event and which trigger a major stress with all the resulting consequences. Inhibition of bowel motility will cause constipation. Abdominal and digestive artery vasoconstriction will result in functional ischemia whose intensity will determine the symptomatology. If the vasoconstriction is limited it will only cause slight clinical manifestations without a lesion visible macroscopically or microscopically at the level of the intestine it is the case of the irritable bowel syndrome.
If the vasoconstriction is more important, there will be ischemia creating a minor cellular suffering and especially in the segments where the intestine is in the remote areas of the irrigation network and especially without anastomosis ie in the areas vulnerable.
This explains the frequency with which the rectum gets affected…
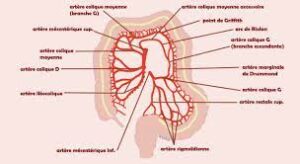
If the cells begin to suffer, they will no longer perform their functions properly, therefore mucus production will be disrupted the physical barrier between the intestine and cells will no longer be as strong as normal and there will be a vulnerability to intraluminal bacteria. In addition, these bacteria will be much more numerous because on the one hand, they will multiply due to constipation and on the other hand the intestinal walls are no longer normal there will be a diffusion of intracellular product to the intestinal lumen creating a culture medium more favourable to bacterial development. And considering the increase of bacteria and the decrease of the intestinal defense, there will be an aggression against the intestine and the process becomes a vicious circle and from there the inflammatory phenomenon will start.
Clinical features
- Clinical symptoms include bloody diarrhea, abdominal pain, mucus discharge, fecal urgency, tenesmus; in severe cases, symptoms may include weight loss, fever or colonic perforation (Mayo Clin Proc 2019;94:1357)
- Characterized by alternating periods of clinical relapse and remission
- At diagnosis, most patients have mild to moderate symptoms, with fewer than 10% having severe disease
- Patients presenting with severe disease are usually those diagnosed at young ages (15 – 30 years of age) or with simultaneous PSC (Lancet 2012;380:1606)
- 30 – 50% of patients will present with disease of the rectum or sigmoid colon and only approximately 20% of patients will present with pancolitis (Lancet 2012;380:1606)
- Appendectomy due to acute appendicitis before age 20 has been shown to be protective against ulcerative colitis (Lancet 2012;380:1606)
- Fulminant colitis, known as acute, clinically severe colitis involving the entire colon and requiring surgical resection, can be seen (Histopathology 2014;64:317)
- Toxic megacolon (marked colonic dilation with signs of systemic toxicity) can occur and requires surgical intervention (BMJ Case Rep 2018;2018:bcr2018227121)
- May have iron deficiency anemia
- Increased risk of hypercoagulability and thrombosis
- Disease severity via endoscopy is stratified as remission, mild, moderate or severe
- Numerous severity indices exist
- Goal of endoscopic remission following therapy
Diagnosis
- Correlation of clinical symptoms with endoscopic and histological examination
- Exclusion of other etiologies for colitis (infection, drug, etc.)
- Colonoscopy with biopsy is essential
- Endoscopic findings include erythema, loss of vascular pattern, granularity, friability and erosion / ulceration
- Often a sharp demarcation between inflammation and normal mucosa (Lancet 2017;389:1756)
- High definition colonoscopy or chromoendoscopy are preferred over traditional white light endoscopy due to higher sensitivity (93 – 97%) and specificity (93%) (Dig Endosc 2016;28:266, Histopathology 2015;66:37)
- Targeted biopsies of mucosal abnormalities and random biopsies at each segment of the colon help determine microscopic extent of disease (Gastroenterol Hepatol (N Y) 2017;13:357, Dig Endosc 2016;28:266)
- Esophagogastroduodenoscopy to rule out upper gastrointestinal tract involvement
Laboratory
- Overall nonspecific
- Markers of inflammation
- Erythrocyte sedimentation rate ≥ 30 mm/h
- C reactive protein > 8 mg/L
- Leukocytosis and thrombocytosis
- Antineutrophil cytoplasmic antibodies
- Fecal calprotectin > 50.0 mcg/g
- References: Pathologica 2021;113:39, Diagnostics (Basel) 2021;11:207
Radiology description
- Magnetic resonance imaging (MRI) and computed tomography (CT) may be useful in identifying bowel wall thickening and ahaustral colon but are not sensitive or specific for diagnosis of acute disease
- Plain upright abdominal Xray can be performed in patients with severe colitis to assess for toxic megacolon
- Mid transverse colon dilation > 5.5 cm (Lancet 2017;389:1756)
- Target or double halo sign can be seen in cases of advanced disease
Prognostic factors
- Colorectal carcinoma is the cause of death in an estimated 15% of inflammatory bowel disease patients; risk factors for developing colorectal carcinoma include:
- Duration of disease (increased risk of up to 2% after 10 years, 8% after 20 years and 18% after 30 years)
- Extent of disease, with pancolitis carrying the highest risk
- Simultaneous PSC, severity of colitis, psuedopolyps, family history of sporadic colorectal carcinoma and male sex
- Risk factors for aggressive or complicated disease include:
- Young age at onset, pancolitis, lack of endoscopic healing, deep ulcerations and high concentrations of antineutrophil cytoplasmic antibodies
- References: Lancet 2012;380:1606, Lancet 2017;389:1756
Treatment
(All treatment with drugs is only symptomatic because they treat the inflammation and not the cause and origin of the disease and the only way to cure the disease is limbic rehabilitation and autonomic nervous system remodeling.)
- 5-aminosalicylate agents are first line therapy for mild to moderate disease
- Corticosteroids
- Patients with moderate to severe disease may require thiopurines or biologic agents (anti-TNF therapy or anti-integrin therapy) (Mayo Clin Proc 2019;94:1357)
- Patients with proctitis only may be treated with topical agents
- Colorectal carcinoma surveillance at 8 – 10 years after the onset of symptoms and fixed interval surveillance every 1 – 2 years afterward (Gastroenterol Hepatol (N Y) 2017;13:357)
- Surgery will eventually be required in 20 – 30% of patients with ulcerative colitis that has become refractory to medical management or who have developed dysplasia or colorectal carcinoma (Lancet 2012;380:1606)
- Total colectomy with ileal pouch – anal anastomosis is preferred surgical intervention
New treatment of inflammatory bowel disease