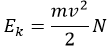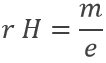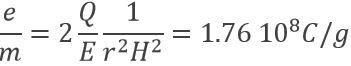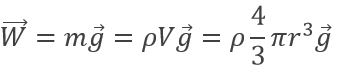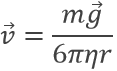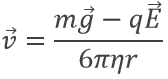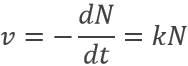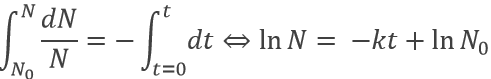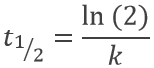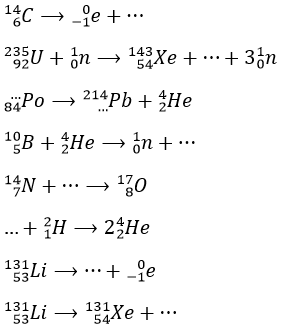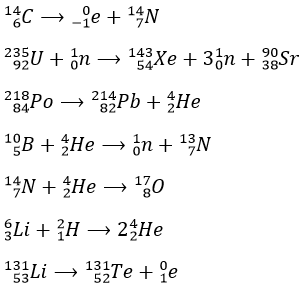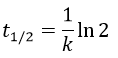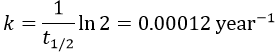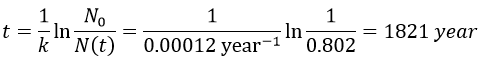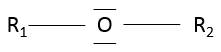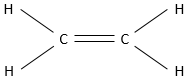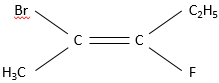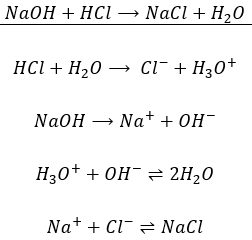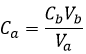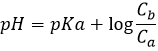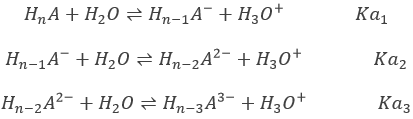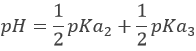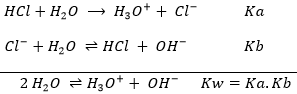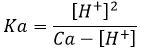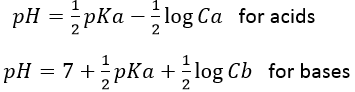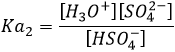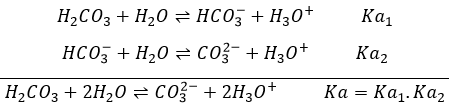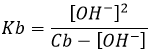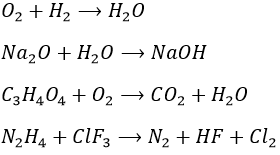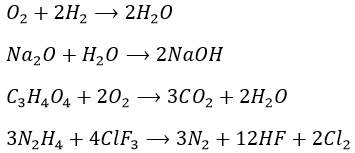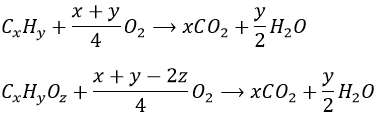Chapter 10 : Atomic theory and nuclear reactions
Atomic theory
At the end of the XIX, it was known that the elements were made of one atom, unbreakable and different for each element. The masses of the atoms were known for several elements but their composition was still a mystery.
Michaël Faraday discovered that atoms were in fact composed of charged species, even if they are electrically neutral. His discovery was the result of an experiment in which a current passes through silver electrodes sunk in a solution containing silver (AgNO3). When the current passes, the mass of the electrode increased significantly. The silver ions in the solutions reacted with the electrons from the current to form solid silver gathering on the electrode.
This reaction showed that atoms contain positively charged elements, and therefore negatively charged species to neutralise the charge of the atom.
Joshep John Thompson proved the existence of electrons in 1897 during his works on cathodic tubes. Those tubes only contain void, a cathode and an anode. If the cathode is heated, a current is detected between the electrodes. The heating determines the kinetic energy transmitted to the atom of the cathode:
While the charge e of the electrons is given by the current:
Applying a magnetic field H on the cathodic tube, if only a small gap allowed electrons to reach the anode, no current was observed: the electron beam deviates from its normal trajectory depending on the ratio mass/charge. The walls of the gap were covered by ZnS, a fluorescent species to detect the deflection of the beam (radius of deflection r):
As a result, the ratio e/m of an electron was determined:
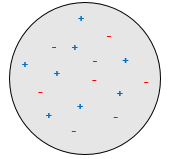
J.J. Thompson imagined a model of a spherical atom wherein a sea of charged species are moving (the plum pudding model).
The charge of the electron was determined by Robert Andrews Millikan. He beamed RX on droplets of oil between two horizontal electrodes. The charged droplets are subjected to several forces: their own weight, the electrostatic force and the friction of the air (air has a given viscosity).
The weight of a droplet is given by
m being the mass of a given droplet, equal to its density multiplied by its volume, and g is the gravity. The electrostatic force is given by
where E is the electric field and q is the charge. The friction is given by
where η is the air viscosity and v is the speed of the droplet. In absence of electric field, the droplet should fall at a speed
resulting from the equations of W and FR. However in the electric field E, the speed of a droplet is affected:
Except q, all the terms of the right side of the equation are known and the speed of droplets was experimentally determined: a droplet is observed through a microscope to measure the time it takes to travel a given distance.
The result of the experiment was that the charge q was always a multiple of 1.602 10-19C, some droplets being several times charged.
The mass of an electron could thus be determined as well: me=9.11 10-31kg.
Despite his brilliant experiment, the plum pudding model of J.J Thompson was not correct and got refuted by Ernest Rutherford (who actually has been one of his student), the pioneer of the nuclear chemistry. Rutherford studied the emission of α particles from Uranium. α particles are the equivalent of the nucleus of Helium atoms: 2 neutrons and 2 protons: He2+. α particles were beamed towards a thin foil of gold. Considering the model of Thompson, all of the radiation should have passed through the gold foil. 99% of it passed, and it was not due to an experimental error. Some of the α particles were deflected in all directions. He concluded that the 1% deflected on a solid aggregation with an intense positive charge and composing the majority of the mass of the element. The rest of the volume of an atom being filled by empty space and a cloud of electrons. The size of an atom is about 1 angström (1Å)=10-10m of diameter and the size of a nucleus is 10-15m.
Rutherford was also the first to transmute an element into another one. He did that by bombarding pure nitrogen by α particles and obtained oxygen and hydrogen nuclei (protons were not yet known). He assumed that hydrogen nuclei are part of the solid nucleus of the atoms. From now, atoms were no more unbreakable.
Later, Rutherford theorized the existence of neutrons to keep the positively charged nucleus in one piece, reducing the repelling of protons and giving a cohesion energy to the nucleus. The bigger the atom, the more neutrons are needed (in proportion with protons).
This energy of cohesion is actually enormous. For example Oxygen is made of 8 neutrons and 8 protons. But the mass of the atom of oxygen is smaller than the addition of the mass of the separated protons and neutrons:
The mass of an oxygen atom is 2.65535.10-23g. The difference is about 2.269.10-25g by atom. As E=mc2, we obtain for one mole an energy of cohesion of 1.23.1013J/mol of oxygen. For comparison, a typical chemical reaction is ~105J/mol. There is thus no surprise why nuclear reactions car produce so much energy.
Nuclear reactions
Nuclear chemistry is a very specific domain of chemistry. It is one domain where the Lavoisier rule does not apply: the elements are not conserved and mass can be converted into energy. Yet, several types of reactions can be sorted. But first, we will take a look at how to write the isotopes involved. We have seen that each chemical elements is an atom with a specific composition of protons, electrons and neutrons. This composition gives the sepcific properties of each element. However, there can be several forms for many elements. Those forms are the isotopes. They differ by their number of neutrons, the number of electrons and of protons being equal and fixed for every element. The chemical properties of isotopes are almost identical (because given by the electrons), but some physical properties can be different between isotopes of the same element. The speed of reaction and the ebullition temperature are two examples of properties that change depending of the isotope. The isotope 238 of Uranium is written
U is the symbol of the element, the mass is written at the top left of the element and its atomic number Z is written at the bottom left. The atomic number can be skipped. The proportions the isotopes of a single element are not equal. For instance, the carbon has 2 stable isotopes: 12C and 13C with a proportion of 98.93% and 1.07%. 14C is an isotope of the carbon but it is not stable: it decays over time. Historians use this property to date ancient items or bodies.
- Production of α particles
Remember that an
The decay of Uranium 238 is an example of reaction producing α particles:
The total value of the top number is conserved during the reaction. The same is true for the bottom number. The γ product is gamma radiation, produced by radioactive decays because the formed nucleus is generally in an exited mode. To come back to its base state, energy is thus freed in the form of an electromagnetic radiation
- Production of β particles
The β particles are small charged species emitted during some nuclear reactions. The Thorium 234 obtained previously can produce this kind of particles
The mass of the element did not change during this reaction. However, the element changed from Thorium to Protactinium. One neutron became a proton. The β particles produced during this reaction is an electron rejected from the neutron to become a proton.
An antineutrino is also generated. A nucleus has a given spin depending on its charge: it turns on itself in a given direction. Electrons also have a spin. During the nuclear reaction from above, the charge of the nucleus changed and an antineutrino is liberated to obtain the correct spin.
A second β particle can be obtained, for example during the decay of Sodium 22
The β particle is not an electron but a positron. A neutrino is also obtained during this reaction. Neutrinos and antineutrinos are radiations that can go through everything.
Electrons and positrons can neutralize to produce a gamma radiation
- Fission
Fission is done by bombarding an isotope with neutrons. In nuclear plants, fission is done on Uranium 235
There is more neutrons produced by the reaction than needed to launch it. This reaction can thus start again as long as there is Uranium 235 in the reactor. Another way to stop the reaction is to trap the neutrons with another isotope.
- Fusion
Fusion is done by merging two isotopes. For example, two isotopes of Hydrogen can produce Helium
![]()
Another way to obtain Helium is to bombard Hydrogen with electrons.
It is also a fusion reaction.
The elements used in fusion and in fission are different. The energy of cohesion is different for each element and we can observe a maximum of energy of cohesion/nucleon (proton+neutron) for 56Fe. Fusion is performed on elements of lower mass up to the 56Fe. Atoms gain energy of cohesion during fusion. On the other side, elements of higher mass lose cohesion energy and mass up to the 56Fe.
Half-life time
Radioactive elements do not stay active forever. The radioactivity decreases over time proportionally at the number or particles.
where v is the speed of decomposition, N the number of radioactive particles and k is a speed constant depending on the isotope.
Because it depends on the number of particles, the speed decreases over time. We can integer the speed equation
The half-life time is the time needed to decrease the population of a radioactive element by half:
This time does only depend on the isotope and does not depend on the population of the isotope.
Exercises
1. Complete these nuclear reactions:
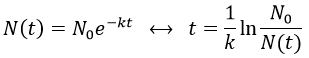
2. A piece of manuscript has been analysed for dating. It has been found that the ratio 14C/12C of the manuscript is equal to 0.802 times the value of a plant of today. Given that the half-life time of 14C is 5720years, what is the age of the manuscript?
3. How much energy is generated by the fusion of 1.2g of deuterium (D or 21H)
given that the masses of the two species are MD=2.0141g/mol and MHe=4.0026g/mol?
Answers
1.
2. The half-life time of a radioactive element defines the time it takes to the element to decrease its population by half. The general formula to calculate the population of an isotope is
The half-life time is
It allows us to determine the value of k:
The ratio 14C/12C that we have in the wording is the reverse of N0/N(t). Now that we know the value of k, we can thus find the age of the manuscripts with the equation
3. In 1.2g of deuterium, there is 1.2g/2.0141g/mol=0.5958mol. We need two deuterium to form one atom of helium, so the amount of helium after the reaction is 0.2979mol.
The energy ΔE generated by the reaction is proportional to the mass Δm lost during the reaction. This mass lost for each produced mole of He is
Thus Δm=0.007626g for 1.2g of deuterium. The energy generated is given by
Chapter 9: Reactions in organic chemistry: Nucleophilic reactions
We will now discuss the types of reactions that can be performed on organic compounds. In this first year lessons, we will only focus on one of the most used reactions: the nucleophilic substitution (SN).
A substitution reaction is indeed the base of the organic chemistry: its goal is to substitute one group of a given molecule by a desired other one. Other important types of reactions are the addition and the elimination (they will be seen in the second year courses most probably).
At the end, we can, in theory, do whatever we want to organic compounds but it is obviously not as easy as said. To this point, you only had the explanation of half of the name of the nucleophilic substitution.
The nucleophile notion is also a very important in organic chemistry. A nucleophile is a compound, rich in electrons, that is attracted by nucleus (poor in electrons) and can share a pair of its electrons to form a liaison. By opposition, an electrophile is a compound, poor in electrons, that is attracted by electrons. On the other hand, acidity as we have seen it previously is not that important. Acidity is important in aqueous solutions and organic compounds do not dissolve well in aqueous solution but are in two separated phases. In aqueous solutions, species are dissociated and in organic solutions, they are not. The separation method takes advantage of that to purify or separate compounds.
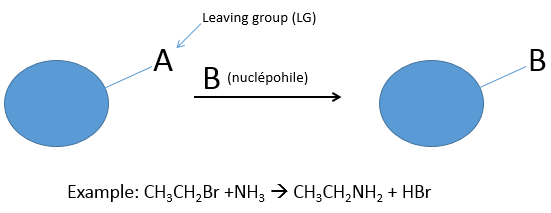
During a nucleophilic substitution, a nucleophile attacks a target molecule to substitute a group, called the leaving group (LG). The target of the nucleophile is a carbon atom poor in electrons because it wears a group of an atom which is more electronegative. It limits the possibilities of targets to halogens and groups with O or N (not nitro groups because the azote has a positive charge there). In the case of SN, the leaving group takes a pair of electrons with itself.
The leaving group is a nucleophile itself and for the reaction to be made, the attacking nucleophile has to be a stronger nucleophile than the leaving group. Several parameters are determining the nucleophile strength (or nucleophilicity).
Charge of the nucleophile
First of all, a nucleophile has not to be charged. Possessing a lone pair of electrons is enough. H2O, NH3, CH3OH are all nucleophiles thanks to their free pairs of electrons (on the O and on the N). However, their negatively charged equivalent is a stronger nucleophile.
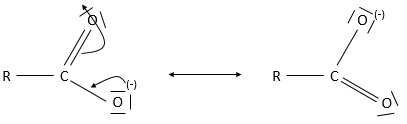
Either the charge or the free pair of electrons have to be available for the reaction. If the charge (or the lone pair) is delocalized, the nucleophilicity decreases significantly. Despite all their lone pairs and their charge, carboxylates are not good nucleophiles because the charge can be displaced. We say that a carboxylate is stabilized by resonance because this compound has a smaller tendency to react with other compounds because of its two resonance forms.
Basicity
Even if the acidity is not so relevant in organic solutions, there is a good relation between the basicity of a compound and its nucleophile strength.
Electronegativity
The nucleophilicity decreases with the electronegativity of the atom wearing the charge/lone pair. Remember that electronegativity is the ability of the atom to keep its electrons. As a result, the electronegative atom won’t share its electrons to form a liaison.
Polarisability
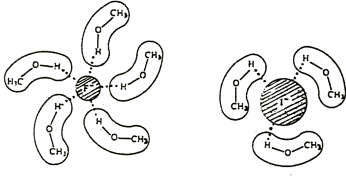
Nucleophilicity decreases with the polarisability of the atom. While it is counterintuitive with regards to the reaction, this relation is due to the solvation of the nucleophile. In protic solvents, the nucleophile species are surrounded by solvent molecule, forming hydrogen liaisons with the lone pairs of the nucleophile. Interestingly, the solvation is stronger for small molecules than for larger ones because the charge density is larger for smaller molecules.
In aprotic solvents, this effect does not exist and the polarisability favours nucleophilicity.
Mechanisms for nucleophilic substitutions
There are 3 possible mechanisms for a substitution:
- First the attack of the substrate by the nucleophile, then the ejection of the leaving group
- Simultaneous attack of the nucleophile and departure of the leaving group
- First the departure of the leaving group and then the attack by the nucleophile.
The first mechanism is quickly ignored because the first step leads to a pentavalent carbon atom. The two other mechanisms exists and are in concurrence. In some conditions, both can occurs in the same time. We will now see the mechanisms in detail and see the differences between them.
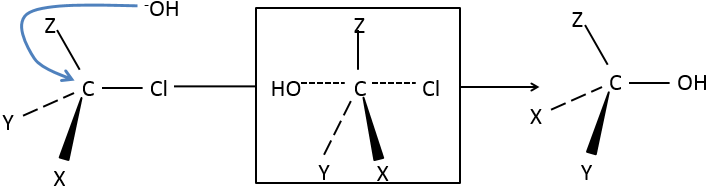
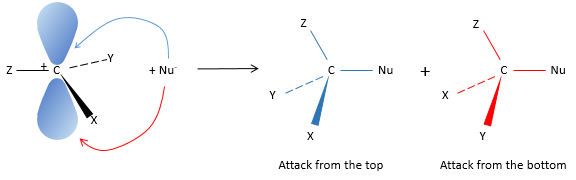
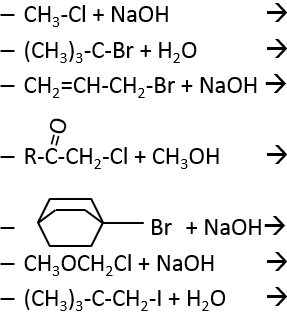
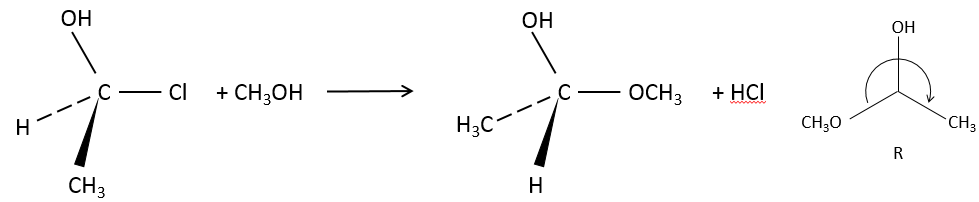
SN2: Bimolecular Nucleophilic Substitution
SN2 is the second mechanism of the list: simultaneous attack of the nucleophile and departure of the leaving group. The whole process is done in one step and the speed of the reaction depends on the concentrations of the two reactants (the substrate and the nucleophile). It is the reason why this mechanism is called bimolecular.
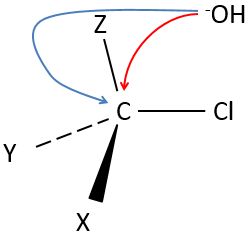
Our first step is to determine where the nucleophile attacks on the substrate. Considering the substitution of a chlorine by an alcohol on a given molecule (X, Y and Z being the rest of the molecule), the nucleophile (OH–) has two options:
- Attack by the side of the chlorine
- or at by the other side
Both sides give the same result in its composition, but the connectivity differs.
Attack by the side of the leaving group (called frontal attack)
To be simultaneous, the liaison between the substrate and the leaving group loosens while the liaison between the substrate and the nucleophile forms itself. It gives place to a transitory complex to obtain next the substituted alcohol.
The connectivity of the formed molecule is identical as the initial one: X, Y and Z do not change of position during the process.
Attack by the other side (called dorsal attack)
Again, a transitory complex is formed. This complex shows differences with regards to the transitory complex observed for a frontal attack: the conformation of the molecule changes during the process. The liaisons between X, Y and Z with C are in a single plane, perpendicular to the “liaisons” with OH and Cl.
As a result, the position of X and Y are inverted. The products of the frontal and dorsal substitutions are chiral products (mirror images from one each other).
We can thus easily determine which results are obtained using the optical activity of each enantiomer. It showed that only the dorsal substitution is done. The frontal attack does not occur.
There are two consequences:
- there is an inversion of configuration after a SN2
- steric hindrance slows or blocks the reaction. The size of X, Y and Z plays thus an important role in the speed of the reaction. For example, the SN2 goes 145x faster for CH3Br than for CH3CH2Br, which goes itself 128x faster than (CH3)2
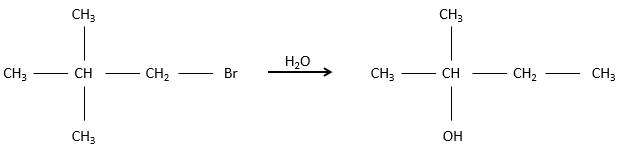
SN1: Monomolecular nucleophilic substitution
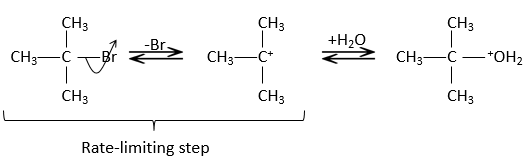
In this mechanism, the departure of the leaving group acts first and then the nucleophile attacks the substrate.
There are thus two steps in this mechanism. The global speed of the reaction is given by the slowest step ‘called the rate-limiting or rate-determining step), which is the departure of the leaving group. Indeed, it implies the breaking of a liaison and a separation of charges. A protic solvent helps to separate the carbocation and the leaving group, through solvation of the charged species. The second step is much faster: the nucleophile is attracted by this carbon wearing the positive charge.
The limiting step is thus the first step of the substitution, where the leaving group is dissociated of the substrate. This reaction implies only one reactant, the nucleophile acting only at the second step of the substitution. The name of SN1, monomolecular nucleophilic substitution comes from there. The kinetics depend then only on the concentration of the substrate, contrarily to the SN2 where the concentration of the nucleophile has an influence on the speed of reaction. A way to determine if a substitution reaction is SN1 or SN2 is to perform a kinetics analysis, determining if the concentration of the nucleophile has or has no influence on the speed of reaction.
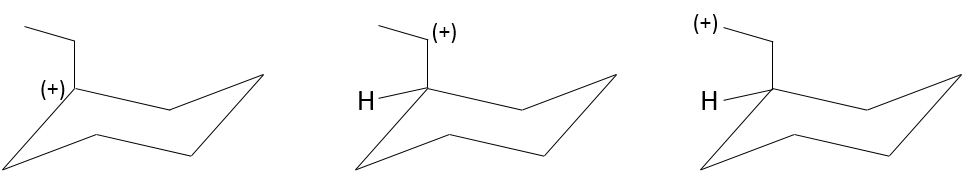
To have a SN1, the positively charged carbon, called carbocation, has to be stabilized.
Carbocation
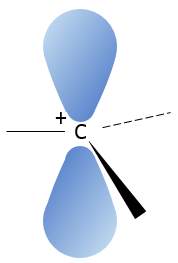
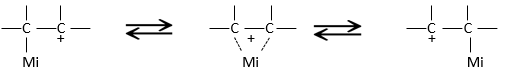
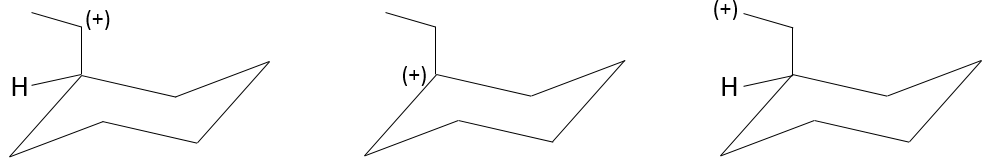
A carbocation is a carbon wearing a positive charge. It has three liaisons in a single plane and an empty orbital perpendicular to this plane.
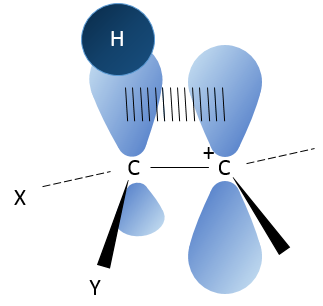
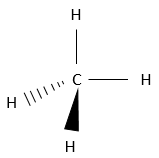
That geometric configuration stabilizes the carbocation with optimized angles between liaisons (nb: plane or quasi plane structure has to be obtained) but also by hyperconjugation. Hyperconjugation is the interaction of the electrons in a sigma bond (usually C–H or C–C) with an adjacent empty (or partially filled) non-bonding p-orbital (the case of carbocations), antibonding σ or π orbital, or filled π orbital, to give an extended molecular orbital that increases the stability of the system. In other words, the liaisons of adjacent carbons of the carbocations stabilize the carbocations when correctly oriented.
In the picture above, one can see the hyperconjugation of a sp liaison between C-H with the empty p orbital of the carbocation.
As a consequence, substituted carbocations are stabilized through hyperconjugation. The sequence of stability for carbocations is thus:
As you have maybe already understand, one parameter to determine if the mechanism of substitution is a SN1 or a SN2 is the substitution of the carbon wearing the leaving group. Trisubstituted carbons lead to SN1 and monosubsituted or unsubstituted carbons lead to SN2. With bisubstituted carbons, both mechanisms can take place, and are pretty slow.
Adjacent π-liaisons also stabilize carbocations through (hyper)conjugation of orbitals and through resonance.
Let’s come back to the mechanism of SN1. Carbocations are plane and can be attacked by both sides of the plane. The proportions of attack on each side are equal except if there is a difference of steric hindrance for one side.
As a result of the SN1, we obtain a racemic melange of two enantiomers.
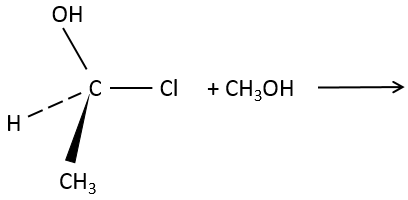
However, we can sometimes obtain very different and unexpected products from a SN1. For example,
This product is quite unexpected: the leaving group was not substituted by the nucleophile but by a methyl while the nucleophile took the place of a methyl not even on the same carbon than the leaving group. The reason why such a product is obtained is that the carbocation produced during the SN1 was not the most stable carbocation that the molecule could have. Through a not totally understood mechanism (called Wagner-Meerwein mechanism), a migrant group (a methyl or a Hydrogen) is displaced to obtain a better carbocation:
If a methyl and a Hydrogen were on the adjacent carbon, the hydrogen is displaced because it leads to a better carbocation
Only then the nucleophile attacks the carbocation.
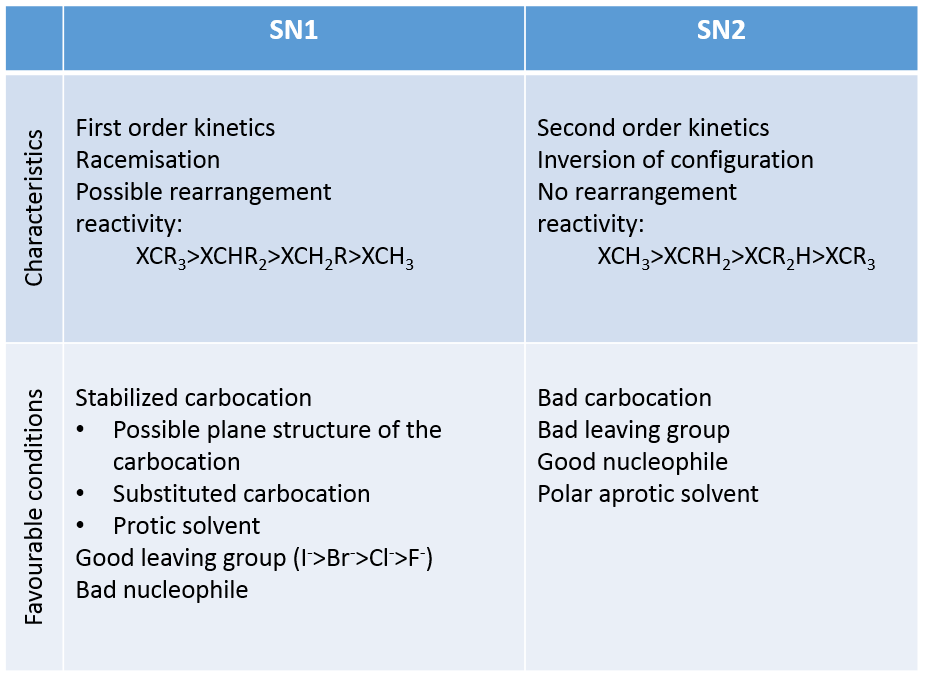
SN1 vs SN2
The mechanisms themselves are not complicated but it may be hard to determine which mechanism takes place. We can resume the parameters influencing the substitution mechanism as follow:
As it was said at the beginning of this chapter, nucleophilic substitutions is one of the most important reaction in organic chemistry. Basically, you will want to substitute a group to extend a chain, to place a desired group on the substrate and to protect a group.
Other substitutions than SN1 and SN2 exist but we won’t talk about them now. They are much less common.
Exercises
1. Sort those compounds from the best to the worst nucleophile:
NaOH CH3COOH N+(CH3)4Cl– SH2 NaI
C4H5N N(CH3)3 CH3OH H2O NH3
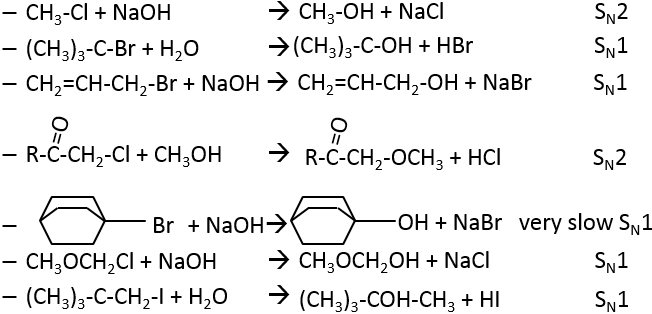
2. Which product and mechanism is used for the following reactions:
3. What is the product and its configuration for the following reaction:
4. Sort those carbocations by stability
Answers
- NaI>NaOH>SH2>NH3>H2O>CH3OH>CH3COOH>N(CH3)3>C4H5N>N+(CH3)4Cl–.
The anions are the best nucleophiles. I- is better than OH- because Iodine has a very high polarisability. SH2 and NH3 can both be compared to H2O. NH3 is a better nucleophile than H2O because N is on the left of O on the Mendeleev table (i.e. more basic). SH2 is a better nucleophile than H2O because S is under O on the Mendeleev table (i.e. more polarisable). Between SH2 and NH3, SH2 is the best. Next come H2O and CH3OH which are weak nucleophiles. Here the size matters. The carboxylic acid is very weak because of its resonance forms. The tertiary amine is very weak as well because the lone pair is sterically hindered by the methyl groups. In the next group, the lone pair of the azote takes part in the aromaticity of the cycle. The last group is not at all nucleophilic: positively charged, hindered and no lone pair of electrons.
2. The reactions are the following:
3. The product is the R enantiomer:
4.From the most stable to the less stable:
Chapter 8: Functional groups
Other than alkanes, alkenes, alkynes and halogenoalkanes, there is a large number of functional groups that can be found in organic chemistry. In this first year, we will only take a look on the richness of possibilities that organic chemistry allows through the functional groups a carbohydrate chain can wear, and of some of their properties. We will not yet talk about their reactivity or how to produce them.
Basically, the major atoms in the living, and in organic chemistry, are C, H, O and N. Functional groups are usually made of O or (/and) N, but sometimes other atoms are to be considered.
O based groups
There is a lot of different functional groups containing Oxygen atoms. If both N and O are in a group, I placed this group in the N based group section.
Oxygen is inductive captor and mesomeric donor. Inductive captor means that it attracts electrons of the adjacent carbons because of its electronegativity. Mesomeric donor means that oxygen can share a lone pair with the rest of the molecule, to eventually form resonance structures.
- Alcohols
Alcohols are molecules wearing a OH group on a carbon.
Nomenclature: add –ol preceded by the position of the group. For example ethanol, propan-2-ol, ethan-1,2-diol.
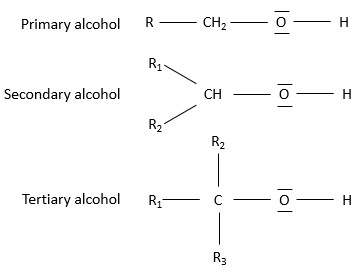
The alcohols can be subdivided in three groups based on the numbers of chains on the carbon wearing the alcohol:
Primary alcohol: One chain is bound to the carbon. The general formula is R-CH2OH.
Secondary alcohol: Two chains are bound to the carbon. The general formula is R1,R2-CHOH.
Tertiary alcohol: Three chains are bound to the carbon. The general formula is R1,R2,R3-COH.
Properties:
Alcohols are weak acids (pKa~16-18). The acidity decreases from primary to tertiary alcohols because the chains share their electrons by inductive effect toward the O. The charge of the conjugate base of the alcohol is thus increased by the presence of chains on the carbon. A larger charge means that the conjugate base is less stable, and will be less probably produced by the dissociation of the proton. Therefore the decrease of acidity. Moreover, the chains increase the size of the molecule, what decreases the solvatation of the ion. That also decreases the acidity of the alcohol.
They are good nucleophiles: The lone pairs of the oxygen can attack electrophile targets.
Alcohols can accept and make hydrogen bonds.
- Ethers
An ether is a group in which an oxygen atom is connected to a second chain: R1-O-R2. Note R1 may be the same than R2, giving a cyclic chain with a heteroatom (=O).
Two nomenclatures are used:
- the names of the chains followed by ether. Examples: dimethylether, ethylmethylether
- the form ‘alkoxyalkane’. The smallest chain is the first noted. Examples: methoxyethane, ethoxypropane
Properties
In general, ethers are volatile and have a low boiling point.
They can be protonated, then form a good leaving group for a SN2. Ethers are often used to protect a group that we don’t want to substitute: form the ether, proceed to the substitution in basic conditions, and remove the ether.
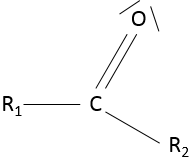
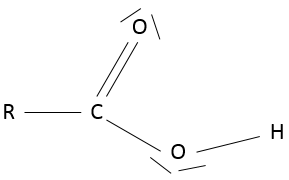
- Ketone
A ketone is a double liaison between a carbon and an oxygen, in the middle of a chain: R1-C(=O)-R2.
Nomenclature: the name of the corresponding alkane with the suffix –one. For example, CH3COCH3: propan-2-one. Note that for this particular compound, a common name is preferred: acetone.
Properties
There is a dipole between the C and O of the ketone. The C is electrophilic while the O is nucleophilic. The oxygen can also accept hydrogen bonds.
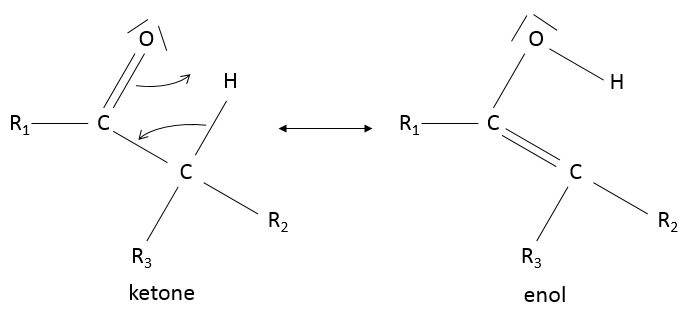
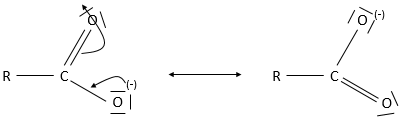
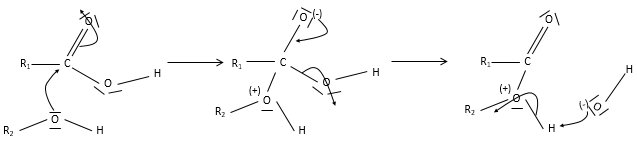
Keto-enol tautomerization: if a hydrogen is on an adjacent carbon of the ketone, a resonance form exist for the ketone, catalysed in acidic of basic conditions
Because of this process, ketones are acidic (pKa~20): the resonance form stabilizes the negative charge of the conjugate base.
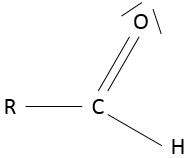
- Aldehyde
An aldehyde is similar to a ketone except that it is at the end of one chain: R-C(=O)-H.
Nomenclature: the name of the corresponding alkane with the suffix –al, for simple alkane chains or –carbaldehyde for more complex molecules. If another functional group is already responsible for the suffix of the name (an alcohol for example), the prefix formyl is used instead. The COH extremity is also called a formyl and the smallest aldehyde, HCOH is called formaldehyde.
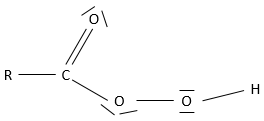
- Carboxylic acid
A carboxylic acid is a ketone bound to an alcohol: R-C(=O)-OH
Nomenclature: the name of the corresponding alkane with the suffix –oic acid. Examples: butanoic acid, propanoic acid. However, there are several common names for some carboxylic acids, derived from what we saw for the previous functional groups: formic acid (HCOOH, from insect stings), acetic acid (CH3COOH), butyric acid (CH3(CH2)2COOH, from butter) and many others.
Properties
Obviously, carboxylic acids are acid. They are more acid than alcohols and very acidic with regards to the other organic compounds. While alcohols have a pKa~16-18, smaller than the pKa of water, the carboxylic acid is a weak acid, with pKa between 3-5. This acidity comes from the resonance forms of the carboxylate, the conjugate base of the acid:
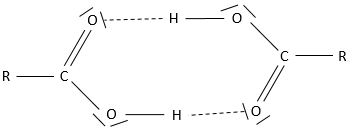
The carboxylic acids are polar and soluble in water (and polar protic solvents), but also in nonpolar solvents: two acids can form a nonpolar dimer by hydrogen bonding.
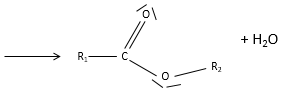
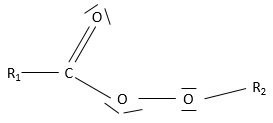
- Esters
An ester is the combination of a carboxylic acid and an alcohol (and it is technically how we can produce esters): R1-C(=O)-O-R2.
Nomenclature: the main chain is the one wearing the carboxylate (R-C(=O)-O-) and is named with the suffix -oate and the rest of the chain is a group. Examples: Ethylethanoate (or ethylacetate), propyloctanoate.
Properties
Esters are polar and can accept hydrogen bonds.
- Peroxides
It is not really a type of group, but more like a modification of existing groups characterised by the bonding of two oxygen atoms.
The peroxide itself is the modification of an ether: R1-O-O-R2.
Hydroperoxide is the peroxide of alcohols: R-O-O-H.
The peracid (or peroxyacid) is the peroxide of carboxylic acids: R-C(=O)-O-O-H.
The perester is the peroxide of esters: R1-C(=O)-O-O-R2.
N based groups
As for the oxygen, the azote is an inductive acceptor and a mesomeric donor.
- Amines
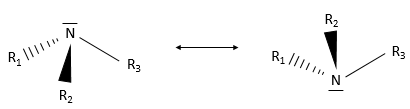
An amine group is an azote atom bound to one or more carbon chains. Primary amines are bound to one chain, secondary amines to two and tertiary amines to three carbon chains. Quaternary amines also exist and wear a positive charge on the azote atom. Secondary and tertiary amines can be found in cycles. So is the piperidine, a very used cyclic amine.
Nomenclature: Whether use the prefix amino- or the suffix –amine. Examples: methylamine, 2-aminohexane.
Properties
Good nucleophiles, depending on the number and the lengths of the bound chains.
Except the quaternary amine, the azote has a lone pair and can oscillate between two conformations.
Amines are bases: the lone pair can accept a proton. The basicity depends on the steric hindrance (and therefore solvatation of the ion) and on the nature of the chains. Alkyl chains are enhancing the basicity (by induction) while aryl chains (an aromatic chain, see next chapter) are decreasing the basicity (by mesomeric donation).
- Nitro group
A nitro group is composed of an azote atom and of two oxygen atoms: R-NO2. It is mainly found on aromatic cycles through electrophilic addition (to be seen next year) and not through nucleophilic substitution.
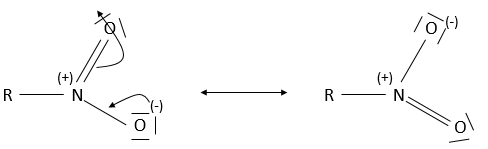
A positive and a negative charge are respectively on the azote and on one of the two oxygen. In fact, there are two resonance forms for the nitro and the oxygen atoms are equivalent.
Nomenclature: The prefix nitro- is added.
Properties
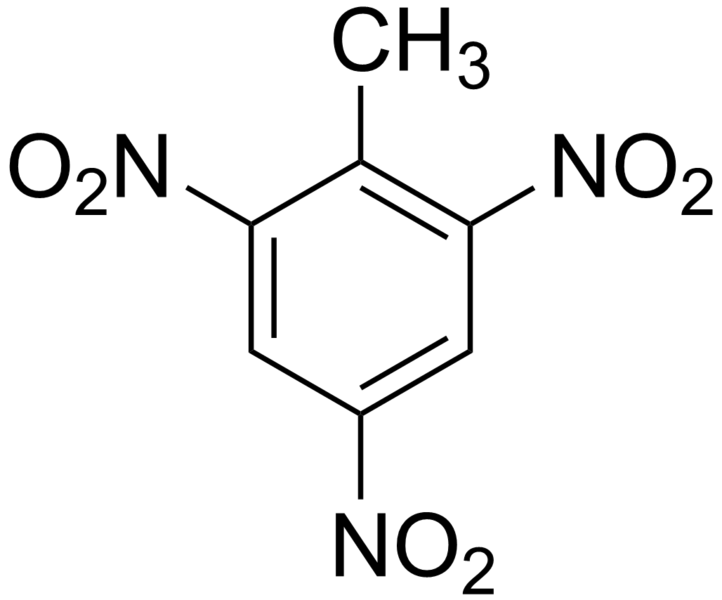
Nitro groups makes compounds explosives. The most known explosive is TNT: Trinitrotoluene.
Don’t heat too much or too quickly a compound containing nitro groups, especially if there is more than one on the substrate.
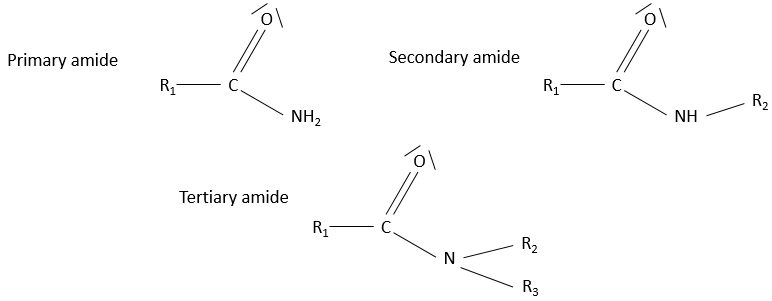
- Amide
Amides are the equivalent or an ester, except that one of the oxygen is replaced by an azote: R1-C(=O)-NH-R2.
Nomenclature: The suffix amide is simply added at the end of the name of the compound. The equivalent of acetic acid is the acetamide.
Properties
Hydrogen’s bonds possible on the azote in both directions. The solubility of amides in protic solvents is good. The solubility is smaller if the azote is bound to 2 carbon chains (tertiary amide)
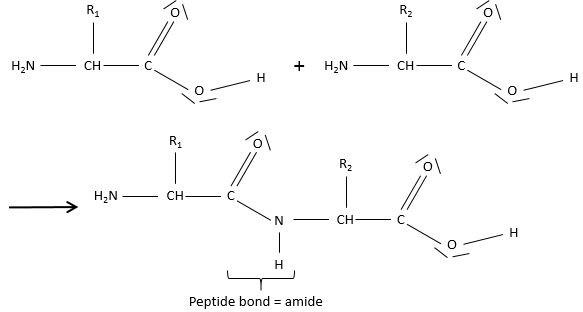
Amides are the bone structure of peptides
They have a resonance structure, separating the charges, similar to the keto-enol tautomerization
- Nitrile
Nitriles are functional group composed of C≡N. In inorganic chemistry, this group is instead called cyanide and is very toxic.
Nomenclature: The prefix cyano- is added to the name of the compound.
Properties
They are used to produce carboxylic acids by hydrolysis, or amines by catalytic hydrogenation.
They are also often used to extend a carbon chain.
The carbon is electrophile in nitriles.
Groups based on other atoms
Some other atoms can be encountered in organic chemistry. We will not develop them a lot in this course.
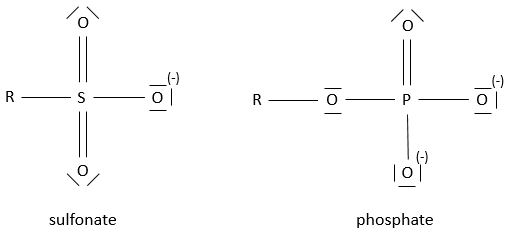
- Sulfonates and phosphates
The prefix sulfo- or phospho- are added to the name of the compound
Chapter 7: cycloalkanes
Cycloalkanes
A cycloalkane is, as it name shows, a cyclic alkane chain. Each carbon of the chain is bound to (at least) two carbons and two hydrogen’s. The general formula is thus CnH2n and the name of the compound is the same name as the corresponding alkane with the prefix cyclo.
Cyclopropane
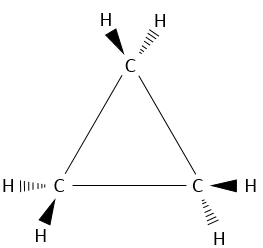
The smallest cycle, cyclopropane, is made of 3 carbons. Each carbon is bound to the two others with a triangle shape.
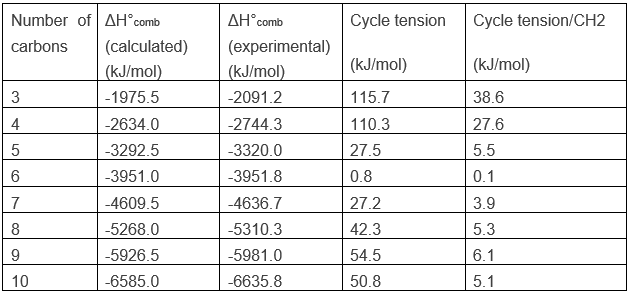
That means that the carbons are in the same plane and that the angle between liaisons is 60°. This angle is far from the normal angle between liaisons in alkanes. Remember that carbons have a tetrahedral structure with an angle of 109.5° between each liaison. To be cyclic, there is a deformation of the carbon structure and a tension of cycle is maintained. It is possible to determine the importance of this tension from the energy of combustion ΔH°comb of the cycloalkane.
For a linear alkane, the heat of combustion increases by approximately 658.5kJ/mol when the length of the chain is increases by one unit. One can conclude that the average ΔH°comb of a CH2 is 658.5kJ/mol. Applying this to the cyclopropane, C3H6, the calculated ΔH°comb is -1975.5kJ/mol. However, when we experimentally perform the combustion, we find that ΔH°comb =-2091.2kJ/mol.
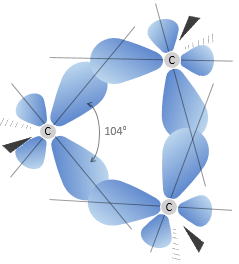
The cyclopropane releases thus more heat than what we could expect. The difference, 115.7kJ/mol (38.6kJ/mol/CH2), comes from the cycle tension, i.e. the molecule requires more energy just to bind this way. In fact, the orbitals of the carbon are not well aligned but the angle between orbitals is 104°.
As a result, the liaisons are weak and cyclopropane is not very stable. It is indeed easily open through catalytic hydrogenation.
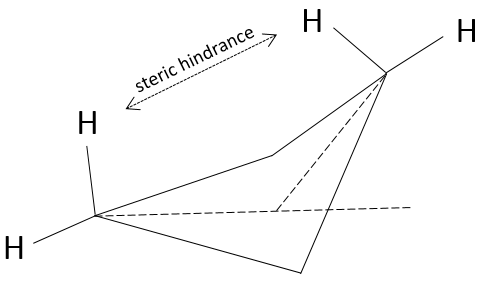
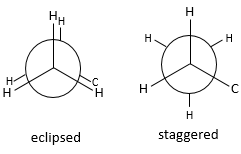
Finally, the position of the hydrogen atoms is unfavourable. Let’s have a quick reminder at the eclipsed and staggered conformations for the hydrogen’s in ethane (C2H6). Hydrogen’s can rotate around the axe formed by the liaison C-C. Each hydrogen has a given volume and feels the atoms in its vicinity (steric hindrance). When rotating, for a given H the distance with the closest hydrogen carried by the other carbon changes.
On the Newman projection,
- the hydrogen’s are on the same spots, or so called eclipsed
- the hydrogen’s are not on the same spots, or so called staggered
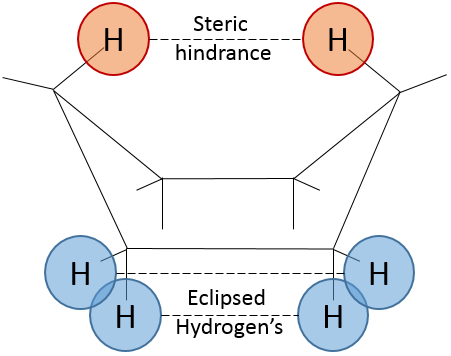
A maximum of energy is reached in the eclipse conformation because repulsion between the hydrogen’s is maximal in this conformation. Cyclopropane has to maintain its hydrogen’s (or its substituents) eclipsed and it requires a lot of energy to the molecule. In the cyclopropane, all the hydrogen’s are eclipsed. The difference of energy between the eclipsed and the staggered conformations can be significant as we will see for cycles of more than 3 carbons.
Cyclobutane
Performing the combustion experiment on cyclobutane, for which the angles are of ~90°, we find an excess of 110.3kJ/mol (27.6kJ/mol/CH2) due to the cycle tension. It is less than for the cyclopropane because the forced bending is smaller in the cyclobutane than in the cyclopropane. The tension for these two cycles are very important. For larger cycles the tension decreases significantly and is at its minimum for a cycle of 6 carbons, the cyclohexane.
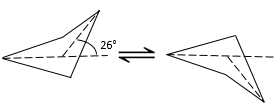
Cycles with more than 3 carbons are not plane. In the cyclobutane, the angle between the fourth carbon of the molecule, out of the plane, and the plane formed by the three other carbons is 26°.
The angle between the carbons is 88.5°. It is slightly less than for a square (90°) in one single plane. So why is the cyclobutane not plane? It should indeed decrease the cycle tension. However, in the plane conformation the 8 Hydrogen’s would be eclipsed. The 26° shaping of the cyclobutane moves the Hydrogen’s out of the eclipsed conformation. The small angle difference is thus counterbalanced by the improvement of the positioning of the Hydrogen’s.
The structure of the cyclobutane is oscillating quickly between two conformers: the carbon out of the plane moves from one side of the plane to the other. These two conformations are equivalent in energy. Looking at the next figure, 4 H are represented.
Two of them (going upwards) are pretty close from each other while the two other are distant. After the oscillation, the roles are inverted and the two Hydrogen’s have in average the same steric hindrance, also called transannular tension in this case.
Cycles can thus oscillate between several conformations when the conformations have a similar potential energy and if the energetic barrier between to switch of conformation is small enough. In the case of the cyclobutane the hindrance is very small, but if one of those 4 hydrogen’s was a substituent, the two conformers are no more equivalent. Indeed, the molecule will place itself in the most favorable conformation which is when the voluminous substituent is not affected by the transannular tension. The proportion of the conformers is no more 50:50.
Cyclopentane
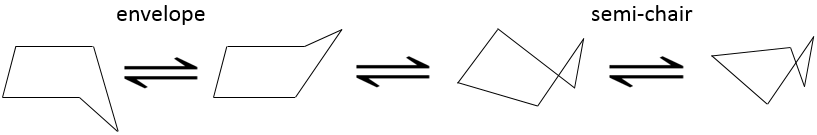
The disfavour of the Hydrogen’s in ecliptic conformations is clear in the pentane. In a regular pentagon, the angle is 108°. It is almost the normal angle for a tetrahedral carbon (109.5°). However, 10 Hydrogen’s would be eclipsing one each other. The cyclopentane, and in fact any cycloalkane except the cyclopropane, is not plane. Two conformations (each with two conformers) are possible: the envelope and the semi-chair.
In the envelop conformation, 4 carbons are in the same plane, with an angle of 104.4°. In the other conformation (semi-chair), the angles are smaller but the eclipsing effect is smaller as well. The two conformations have very near potential energies and the barriers to switch between two forms are easily passed through. Cyclopentane oscillates then quickly between its conformers.
Cyclohexane
The case of the cyclohexane is particular. When we look at the heat of combustion of this species, the experimental value is less than one (0.8) kJ/mol different from the calculated one based on the number of CH2 in the molecule. Cyclohexane is the most stable existing cycloalkane.
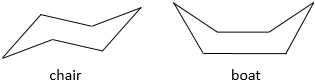
Two conformations exist but one is more stable than the other one.
The most stable conformation is the chair conformation.
In this conformation, 2 pairs of carbons are in the same plane and the last 2 carbons are in each side of the plane. This position is called chair: the 4 carbons on the plane make the seat, one plane of 3 carbon makes the back of the chair and the other makes the footrest.
The angle between carbons is 111.4°, i.e. almost the 109.5° of a normal tetrahedral carbon, and all the Hydrogen atoms are in a staggered conformation.
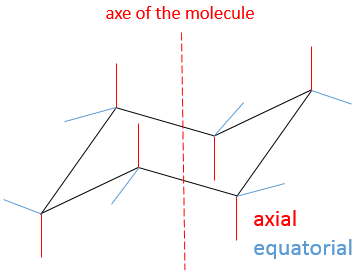
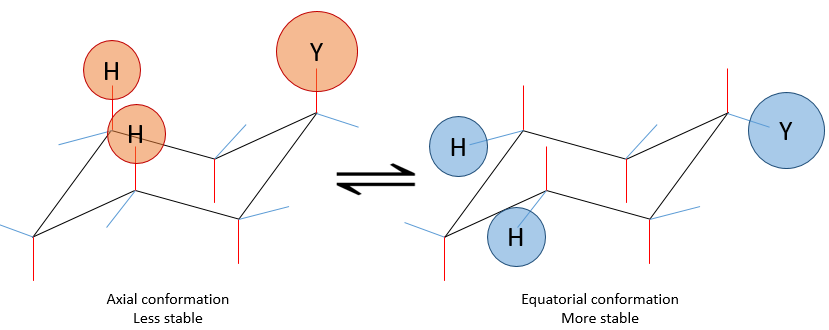
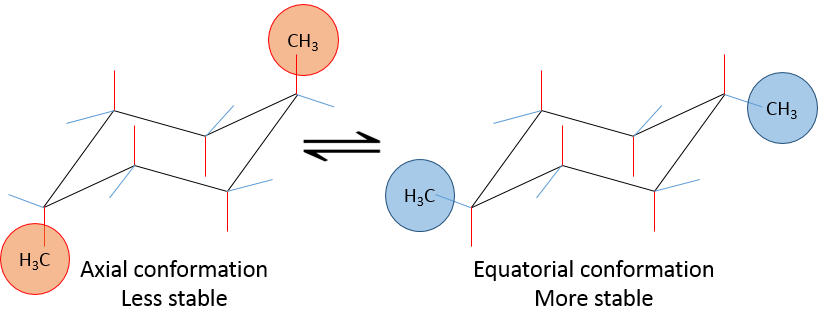
This structure is thus very stable. Two types of Hydrogen’s can be distinguished: the ones in the axial position and the ones in the equatorial position. 6 liaisons C-H are parallel to the axe of the molecule (axe passing in the middle of the molecule). These are the axial Hydrogen’s. The other 6 liaisons are almost perpendicular and are called equatorial.
In we reverse the chair structure (chair’s back<->footrest), equatorial hydrogen’s become the axial ones and vice versa.
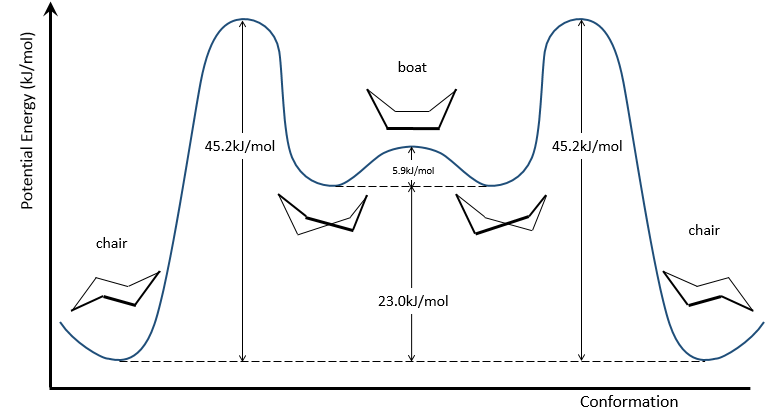
To reverse the chair, cyclohexane has to go through the boat conformation, less stable by 28.9kJ/mol (the energetic barrier is 45.2kJ/mol). In this conformation, the two carbons that were out of the plane are now at the same side of it. This does not only generate a steric hindrance, but the hydrogen’s on the 4 carbons of the plane are now eclipsing each other. That explain the difference in potential energy between the boat and the chair formations.
In reality, this conformation is only a transition state. A more stable form is the crossed-boat conformation, almost identical but reducing the transannular tension. The boat conformation is thus the transition state between the two crossed-boat conformations.
We can resume the conformations as follow:
Cyclohexane in the boat conformations only exist in very small proportions with regard to the chair conformation. We will thus only focus on the chair conformation for our further analyses.
Presence of substituents on the cyclohexane
The positions of a substituent on the cyclohexane are not equivalent.
If a substituent is placed on an axial position, the steric hindrance is greater than on an equatorial position. Indeed, equatorial substituents are more spaced than axial ones which go in the same direction. This is called the 1,3-diaxial interaction. The equatorial position for a substituent is then more stable than the axial one and one conformation of the molecule is favoured. For example, if the substituent is a methyl, the difference of energy between the conformations is 7.1kJ/mol, leading to a proportion of 95:5 (equatorial/axial). Larger substituents increase furthermore the proportion of conformers.
The Newman projection can help to visualise this phenomenon. The whole molecule is represented, connecting two Newman projections together.
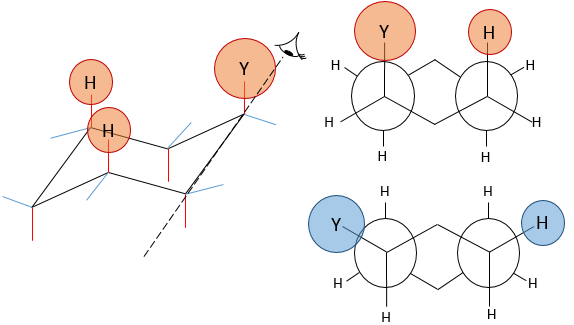
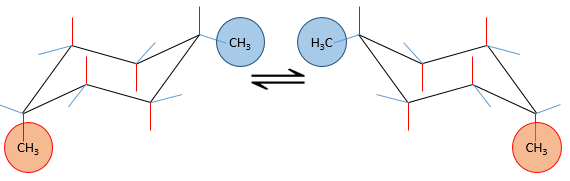
Several substituents may be bound to a cyclohexane. The influence on the stability of each one is, for most of the substituents, simply additive. In the case of two substituents, one will be placed in an equatorial position anyway because it requires less energy than on an equatorial spot (as we just saw for the case of a single substituent). This substituent is the biggest one to minimize the steric hindrance. The second one, smaller, is either on an axial or an equatorial spot, depending on the connectivity of the molecule. If both groups are in equatorial positions, the equilibrium is further balanced towards this conformation. If the second substituent is on an axial spot, the equilibrium moves toward the 50:50 composition, the effects of the substituents acting against one each other.
Let’s see it through some examples. As explained before, the presence of a methyl on the cyclohexane favours the equatorial conformation by 7.1 kJ/mol. The presence of a second methyl group on an equatorial spot will increase the proportion of the equatorial conformation by the same amount (7.1kJ/mol). The total energetic advantage of the equatorial conformation reaches 14.2kJ/mol and the proportion of the equatorial conformer increases furthermore towards 99:1. This conformation is also noted trans because the groups point in opposite direction. The name of this molecule is indeed trans-1,4-dimethylcyclohexane.
If the second methyl group was on an axial spot, the two conformers are equivalents (the axial methyl becomes equatorial and vice versa). The total energetic advantage of one of the conformer is indeed 7.1kJ/mol-7.1kJ/mol=0kJ/mol.
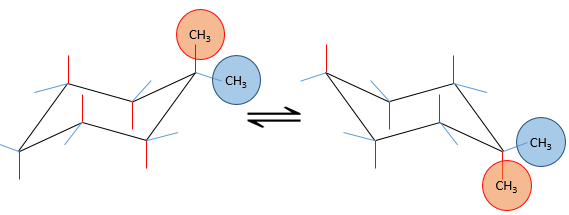
If the second group was a chlorine atom instead of a methyl group, we proceed the same way to know which conformer is favoured. A single chlorine group stabilizes the equatorial conformation by 2.2kJ/mol. If both substituents were equatorial, the total energetic advantage of the equatorial conformer is 7.1kJ/mol (from CH3) +2.2kJmol (from Cl)=9.3kJ/mol. This molecule is thus more stable than the methylcyclohexane.
If the chlorine atom was axial, the total energetic advantage of the conformer wherein the methyl is equatorial is 7.1kJ/mol-2.2kJ/mol=4.9kJ/mol. The conformer with an equatorial methyl is still more stable than the other conformer but less than the methylcyclohexane.
Note that other interactions may affect the stability of the conformers. When the substituents are on other spots than 1-4, interactions (as steric hindrance, repulsion,…) may decrease or increase the energetic advantage of one conformer on the other one.
Polycyclic alkanes
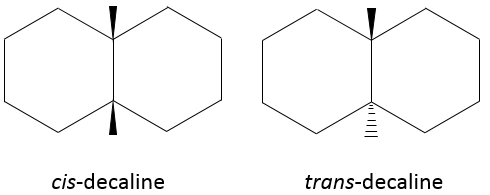
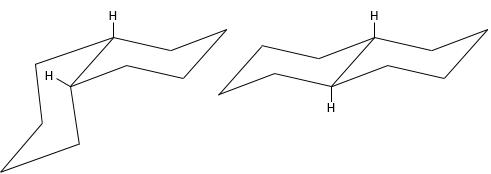
Molecules are not limited to one cycle. Several cycles may share carbons. A molecule composed of two hexanes sharing two carbons is called decaline and exists in a trans and a cis form. Cycles do not have to be the same size and a cyclohexane can merge with a cyclopentane.
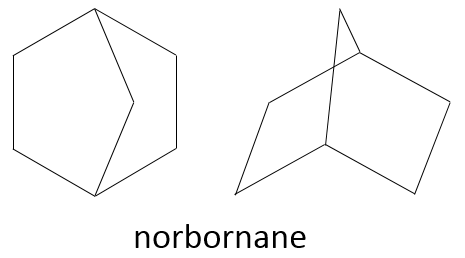
Two cycles may also be slotted one into each other. This leads to bridged cycles. The norborane is a bridged cyclohexane or two cyclopentane sharing 3 carbons. Carbons wearing the bridge are called bridgeheads.
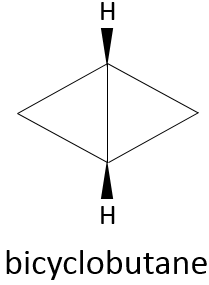
Polycyclic alkanes reduce the freedom of move of the molecule but it seems that there is no limit to the cycle tension that hydrocarbures can endure, as one can think from the bicyclobutane.
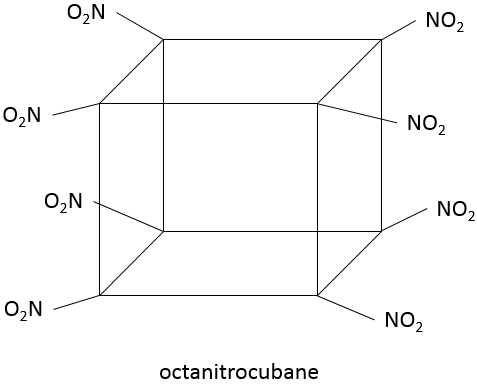
Polycycles with cycles of few carbons (3-4) are in general not naturally produced but may be made through synthesis. All types of carbon skeletons have been made in laboratories. They can have an interest as explosives if they wear nitro croups thanks to their important cycle tension.
Polycycles of larger cycles are often found in natural compounds, giving specific odors, parfums, colors, or very specific roles as do have hormones.
Chapter 6: alkenes and alkynes
Alkenes
Alkenes are organic compounds made of carbons and hydrogens. Opposite to alkanes, which have the same components, the general formula of alkenes is not CnH2n+2. Into an alkene, two carbons are bound by a pi liaison (also called double liaison). The smaller alkene is the ethene CH2=CH2.
To make this liaison, each carbon shares 2 electrons, one for the sigma liaison and one for the pi liaison. There are thus two less hydrogens on an alkene than on an alkane in only one pi liaison is present. The general formula is thus CnH2n for a monoalkene and the number of hydrogen is reduced by two for each additional pi liaison.
Propene is CH2=CH-CH3 and CH2=C=CH2 is the allene.
For larger molecules, the position of the alkene has to be signified in the formula. The alkene is related to two carbons of the chain and is always in the main chain, even if a longer chain could be found.
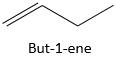
For example,
Is the but-1-ene. The nomenclature is thus that the suffix –ane from the alkanes is removed and replaced by the suffix-ene, itself preceded by the position of the carbon wearing the functional group.
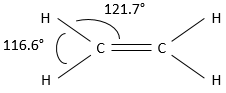
A carbon sharing a double liaison is bound to three other atoms, and not 4. As a result, the tetrahedral structure of the carbon is no more the most stable structure to adopt in the case of alkenes. To have an optimal distance between the atoms/liaisons, all three liaisons are in the same plane. The angle between liaisons is close to 120°. The double liaison takes just a bit more space that the simple liaisons.
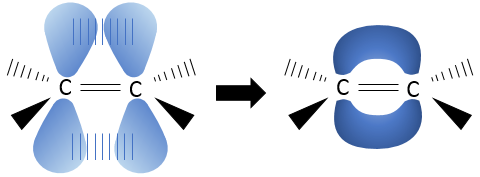
The pi liaison is less energetic than a sigma liaison but is strong enough to forbid the rotation of hydrogen atoms around the axe C=C. The pi liaison is a liaison between sp2 electrons. Those electrons are located perpendicularly of the plane of the molecule.
To form the pi liaison, the electronic orbital sp2 must be aligned. Otherwise the orbital would not superimpose together and would be too distant to form the pi liaison. The rotation is thus no more possible.
It also means that the atoms/chains connected to the double liaison stay in a fixed position with each other. And that we can specifically name the compounds based on their connectivity.
Let’s take an example to explain how to name alkenes with substituents on the double liaison.
This alkene wears a Bromine, a fluorine, a hydrogen and the rest of the carbon chain. To name this substance, we look at the atoms directly connected to the carbons wearing the pi liaison. A priority is determined for each of the two carbons. It goes to the atom with the larger atomic weight. In this case, Br>F>C. Two substituents are made of carbon. If a a priority was to be determined between them, we look at the atoms they are wearing and, again, the priority goes to the carbon wearing the atom of larger atomic weight. The priority is thus Br> F>C2H5>CH3.
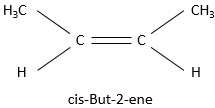
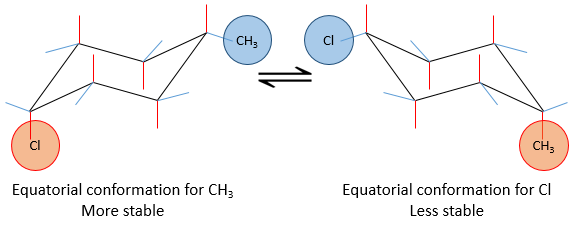
Substituents are in cis position when the h igher priority substituent of each carbon are at the same side of the pi liaison. Otherwise the position is trans.
This alkene for example
is called cis-But-2-ene while the trans-But-2-ene is this molecule
However this method is limited and a more general method is to be used in the case of more complex molecules as our example. We use the E,Z system. The priority on the left carbon is Br>CH3. On the carbon of the right, F>C2H5. If the subsitutes of higher priority are at the same side of the double liaison, the molecule has a configuration Z (for Zusammen, together in German). If they are on the opposite sides the configuration is E (for Entgegen, opposite in German).
The molecule of our example is thus named (E)-2-Bromo-3-Fluoropent-2-ene.
Alkynes
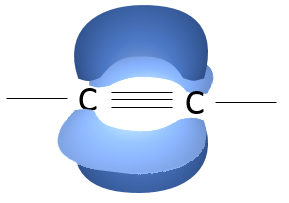
Alkynes are characterized by a triple liaison: one sigma liaison and two pi liaisons. They have a linear geometry.
A chain possessing one pi liaison (double or triple) can be called monounsaturated, possessing two of them – biunsaturated-, or many -polyunsaturated. We often hear these terms about fatty acids from butter, oil or food. Having a high degree of unsaturation limits the mobility of a chain and different chains slide along each other with less friction than between saturated chains. It makes unsaturated substances more malleable than saturated ones. For example, unsaturated oils are less viscous than saturated oils.
Chapter 5: Organic chemistry, structure and names of alkanes
Organic chemistry is the chemistry of carbon and its compounds. Carbon is one element of the Mendeleev table among many others, so why is there a complete section of chemistry related to this particular element? Carbon has a valence of 4 and can thus bind with up to 4 other elements of the periodic table. To that point there is nothing extraordinary. However, where in the inorganic chemistry atoms can only bind together to form small molecules, carbon based molecules can form long and stable chains with a rich variety of functional groups.
Organic chemistry is so called because carbon is the essential constituent of living species: proteins, ADN, lipids, sugars or fats are a few example of organic compounds, made of a structure of carbon wearing functional groups allowing them to interact together, becoming more than just their simple addition but to form a functionning macrosystem where each molecule has a specific role to sustain a stable living body.
Organic chemistry is thus very important in the science of the living, but is not limited to that. Plastic is an organic compound that we find everywhere, oil, toothpaste, shampoos, clothes, deodorants, etc. are products of organic chemistry.
To adventure ourselves in this vast world that is organic chemistry, we will first discuss over alkanes, their structures and how scientists call them, and how they are represented. Later we will introduce the different functional groups that we can find on organic compounds and finally how organic compounds react, how we can produce them or modify them.
Alkanes
Alkanes are compounds only composed of carbon and hydrogen atoms. Hydrogen has a valence of 1, meaning that it can only make one liaison with another atom. A single carbon will thus bind with 4 hydrogen to form a neutral species CH4. This molecule is called methane and is a gas in normal conditions. The liaisons are covalent liaisons. The carbon is slightly more electronegative than the hydrogen but it is not important at this moment of the lesson. Just remember that CH4 will not dissociate from a proton and is not an acid.
There are several ways to represent this molecule. The fully developed representation is as follows:
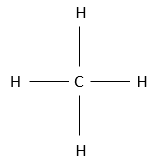
In this representation of the methane, all the liaisons are shown by a full line connecting the atoms. All the atoms are shown independently as well. This representation is in two dimensions and the hydrogen atoms in 3D are in reality not on a single plane. The structure of lowest energy is the structure where the hydrogen’s are the most separated from each other. Indeed, hydrogen’ss have a given volume and are repelling each other. To obtain that structure, an angle of 109.5° separates the liaisons. It leads to a tetrahedric structure.
Most of the time, there is no point in showing the complicated structure of large molecules. It would only make it harder to see the important information of the molecule. However, it is sometime important to know in which direction goes one particular liaison. In this case, the lines representing the liaisons take different forms depending on their orientation. In the plane of the page, liaisons are still represented by a simple line. Two other cases are possible: Liaisons can go in the direction of the reader or in the opposite direction. Liaisons going toward the reader are represented by a black triangle one peak of which is connected to the atom on the plane of the page and the two other points are connected to the atom out of the plane. This way, the triangle looks like a line going wider from the atom in the plane to the atom closer to the reader.
For liaisons going in the opposite direction, atoms are connected by traits (parallel or perpendicular to the liaison direction your choice). For methane, it gives the following 3D representation:
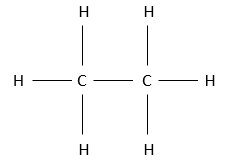
The alkane possessing a structure of 2 carbon atoms is the ethane. It is a gas as the methane. To form the “bone” structure of the ethane, the two carbon atoms bind together through a covalent liaison. Obviously they have the same electronegativity. From their 4 electrons, one is thus used by each carbon to bind with the other one. 6 hydrogen will thus complete the structure. As in inorganic chemistry, the octet rule is respected: to be stable, a carbon has to have 8 electrons (an octet) around it: its 4 electrons and 4 electrons of the other atoms with which it shares a bond. Each carbon of the ethane is thus bound with a carbon and with 3 hydrogens. It is the single possible structure for such compound. In no way one carbon would wear 5 hydrogens and the other carbon 1 or more.
The fully represented structure of the ethane is thus
If we want to represent it in 3D, it would be:
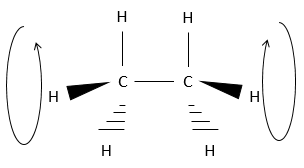
However, it is to be said that atoms can rotate in the axe of a simple liaison. The 6 hydrogen are thus turning in circle around the axis made by the two carbons, as shown above. The Hydrogen’s are rotating almost freely around this axis.
Each hydrogen has a given volume and feels the atoms in its vicinity (steric hindrance). When rotating, the distance between hydrogen’s on a same carbon is constant. However, the distance with the closest hydrogen carried by the other carbon changes.
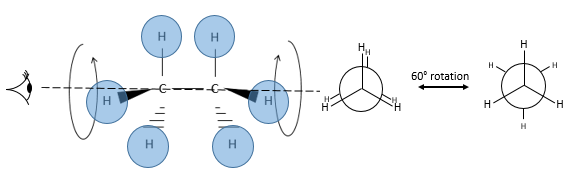
The relative positions of substituents can be showed through the Newman projection. The molecule is observed along its C-C axis. The first C (proximal carbon) is represented by a circle from the centre of which three lines are going out. These lines are the liaisons of this carbon. The second carbon (distal carbon) is hidden by the first one but one part of the liaison is visible.
Fixing the hydrogen’s of the proximal carbon, only the hydrogen’s of the distal carbon can move. Two cases can be observed:
- The hydrogen’s of the distal and of the proximal carbons are on the same spots, or so called eclipsed
- The hydrogen’s are not on the same spots, or so called staggered
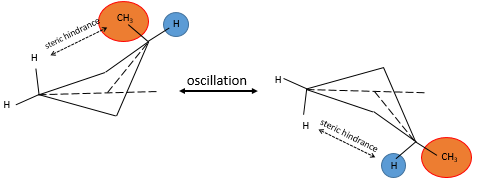
A maximum of energy is reached in the eclipse conformation because repulsion between the hydrogen’s is maximal in this conformation. A rotation of 60° from this conformation leads to a minimum of energy, the hydrogen’s being as far away from each other as they can be. A molecule that has to maintain hydrogen’s (or substituents) eclipsed has a higher energy than a molecule of same composition with staggered hydrogen’s. The difference in energy here is not very important and the rotation is effective. During the rotation, the molecule passes more time in the staggered conformation (smaller energy). If substituents were on the molecule, the steric hindrance increases with the radius of the substituent. In some cases, the rotation can be blocked by the presence of voluminous substituents.
The semi representation of the ethane is
In this representation, the carbons are regrouped with the atoms they are wearing but not sharing. The liaisons between C and H are thus not showed in this representation. If a Hydrogen atom was replaced by a chlorine atom, for example, the half representation would be:
Adding a third carbon atom to the chain, C3H8, the propane, is still a linear alkane. The structure in triangle where each carbon binds with two other carbon exists but is not very stable. You maybe have spotted that the formula of linear alkanes has a general model: CnH2n+2. For each atom of carbon added to the first one, 2 hydrogen are to be added.
There are two ways now to add a fourth carbon to obtain a butane molecule. The chain can be extended by its extremities or by its middle. When the chain is linear, we add n- before the name of the compound. n-butane is thus
![]()
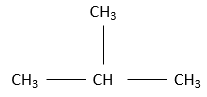
If the chain is extended by its middle, we name this compound isobutane
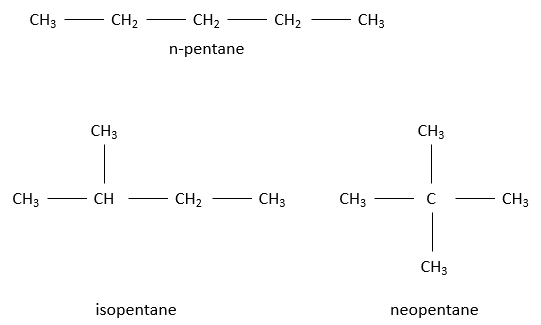
The iso prefix is used only for a few compound wherein a carbon wear two terminal CH3. n-butane and isobutane share the same formula C4H10 but don’t have the same structure. Such kind of compound is called an isomer of constitution. The greater the number of carbon in an alkane, the greater the number of isomers.
For a 5 carbon chain, the fifth carbon can be added at one extremity of the n-butane to obtain n-pentane, equivalently at any extremity of the isobutane (it is the same as adding the C on one CH2 of the n-butane) to obtain isopentane, or on the CH to obtain neopentane.
Names of the alkanes:
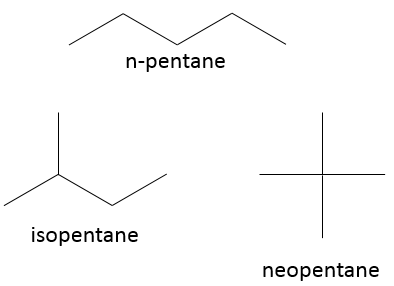
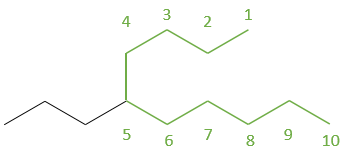
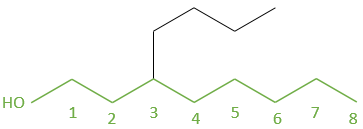
Another representation of organic molecules is the skeleton representation. In this representation, carbons and hydrogens are not shown. The liaisons between carbons are still shown as full lines and are connected together with an angle at the position of the carbon atoms. Without angle, we could not differentiate a chain of 6 carbons from a chain of 7. Generally, the angle is approximately 120° so that if a carbon is bound to three other species (other than H), each liaison is equally distant. If a carbon is bound to 4 species, the angle is 90°.
For example, the pentanes showed above are represented
This bone structure is the representation that is usually used. Only important informations are shown. The number of hydrogen on the different carbons of the bone are determined by the number of liaisons that the carbon has. It is thus pointless to show it. Moreover, this representation is faster to write and takes less space.
There is a given method to name organic compounds. An alkane as a functional group has the same name except that the -ane is replaced by -yl. For example, isobutane is also called methylpropane because a methyl is fixed at a linear chain of 3 carbons, i.e. a propane chain.
C4H9Cl is a Chlorobutane. With this name, we know the components of the compound but not its complete structure. The connectivity in the butane and between the butane and the chlorine are not known.
The rules to name a compound are
- the longest chain is the main one. However if a functional group is on one chain, the main chain has to wear it.
- tag a number to each of the carbon from one side of the main chain to the other. The carbon wearing a functional group which is the closest of an extremity has to have the smallest number.
- Next, we name the compound by writing first the groups out of the main chain, with their number as prefix, in the alphabetical order, followed by the main chain with its group.
- our examples are thus named
- If several identical functional groups are on different carbons, the prefix are separated by a , and their number is indicated by bi, tri,…
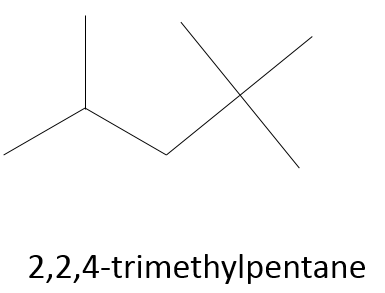
- ex: the isooctane is the 2,2,4-trimethylpentane, meaning that a total of 3 methyl groups are on a main chain of 5 carbons. Two methyls are on the carbon #2 and one on the #4. It is 2,2,4-trimethylpentane and not 2,4,4-trimethylpentane because we favor the carbon wearing more groups.
Halogenoalkanes
Simply said, halogenoalkanes are alkanes wearing one or more halogen. It is simply said but halogenoalcanes are not so easily made. They are made from a dihalogen and an alkane through a radical reaction during which a proton has to be removed from the alkane. This step of the reaction is not favorable but can be made through heavy heating (300° for the chloromethane). Also, the position of the halogen is not completely fixed. The proton removed during the reaction is easier removed from a carbon in the chain than at an extremity but the high temperature makes both positions possible (the distribution depends on the temperature)
Halogens have a higher electronegativity than C and they generate a dipole from C to X. A carbon wearing an halogen is thus poor in electrons and will consequently be targeted by reactants rich in electrons. The reactivity of halogenoalkanes will be seen in a further section.
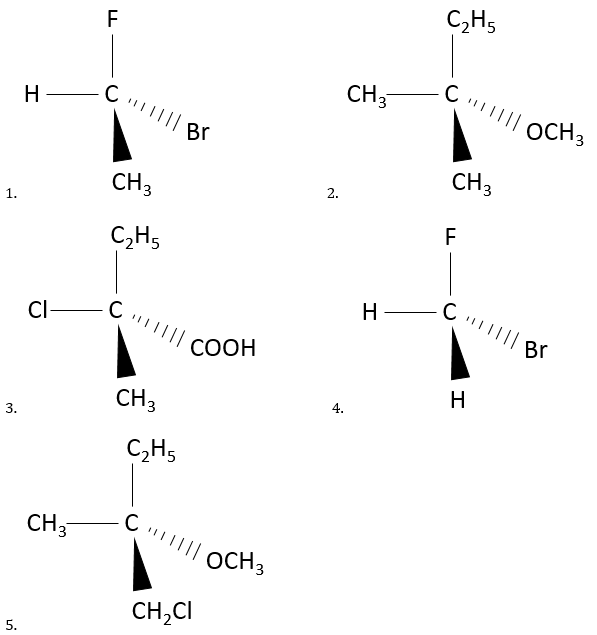
Chiral molecules
We have already see that for one given formula, several different molecules may exist. When the connectivity differs, these are isomers of constitution.
Ex: butane and methylpropane, ethanol and methoxymethane.
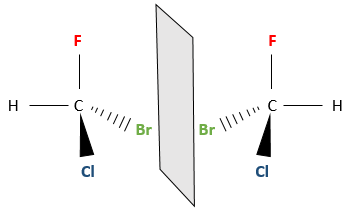
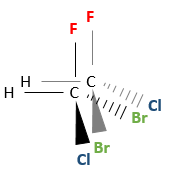
Even on a single carbon, the connectivity may change. Stereoisomers are isomers of same connectivity but with different spatial positioning. The bromochlorofluoromethane has two stereoisomers forms.
These two molecules are mirror images one of the other. It is said that they are chiral if the molecule and its mirror image do not superimpose. These particular stereoisomers are called enantiomers.
A good way to explain chirality is to look at our hands. The left hand is the mirror image of the right hand (and vice versa). However, we cannot superimpose them.
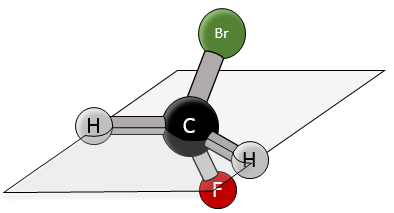
Chirality is related to the carbon that wears several different groups. It is a stereocentre. Stereocentres are often indicated by an asterisc. If a plane of symetry exists for the molecule, this molecule is achiral (><chiral) and this molecule and its mirror image can superimpose somehow. For example, Bromofluoromethan is achiral because a plane of symetry can be drawn, passing by the two hydrogen atoms.
Our body is able to distinguish enantiomers from each other. For some medicines, one enantiomer is active while the other one will do absolutely nothing, or will be less effective. In some cases, it is thus very important to be able to produce selectively one enantiomer and not the other one. Pharmaceutical indrustries invested such methods. If the reaction is not enantioselective, the productivity immediately drops by 50%. The optic activity of enantiomers also differs and is a good way to know which enantiomer have been produced.
Optic activity
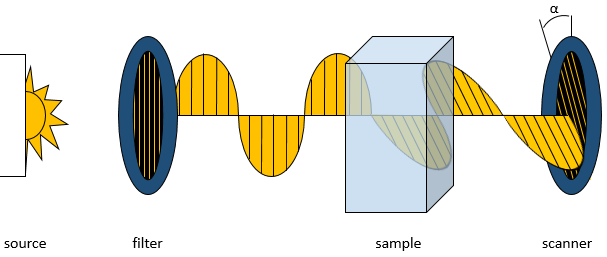
The optical activity of a compound is its influence on a plane polarised light beam. When the light, filtered only to oscillate in one plane, passes through a sample of an optically active compound, the beam is rotated by a given angle.
The angle of rotation depends on the molecules in the sample, of their concentrations and on the length of the sample cell. Each of these effects are linear and the modification of the angle of the light is given by the formula
The interest here is that enantiomers don’t have the same optical activity. The absolute modification of the angle is identical, but the direction in which the light is rotated is not. One enantiomer deviates the light towards the right and the other enantiomer deviates the light by the exact same angle but towards the left. The enantiomers are respectively defined as dextrogyre and levogyre and noted with a (+) or a (-).
The optical activity of an enantiomer is fixed for this molecule, at a given temperature t and for a light of given wavelength λ. Knowing its value, it is possible to determine the quantity of each enantiomer in a racemic mix/melange. A racemic melange is a solution containing the two enantiomers, not necessarily in the same quantity (nb: a more global definition of a racemic melange is the melange of several possible products of a single reaction). In such a melange, all the species will deviate the light with their normal effect.
If the two enantiomers are in equal quantities in the solution, the sample will be optically inactive, the effect on one enantiomer being counterbalanced by the effect of its specular image (i.e. the other enantiomer). If the quantities are not equal, the sample is optically active and the relative quantities of the enantiomers can be determined. The enantiomeric excess is the difference of proportion of the two enantiomers and is practically the proportion of the enantiomers that have an effect on the light. For example if the ratio between the enantiomers was 3:1 (75% of one (let’s say the dextrogyre) and 25% of the other enantiomer), the enantiomeric excess is 50%. Indeed, from the 75% of the dextrogyre enantiomer, the effect of 25% is counterbalanced by the levogyre enantiomer present in the solution. Only 50% of the (+) enantiomer deviates the light beam. If the pure enantiomer would have rotate the light plane by 26°, an entantiomeric excess of 50% rotates the light by 13° (50% of 26°).
Name of the enantiomers
We need a way to name differently the enantiomers. R or C will precede the name of the molecule and we will now see how to attribute which letter to which enantiomer. It is unfortunate but there is no clear correlation between the optic activity of an enantiomer and its structure. Another method had to be find.
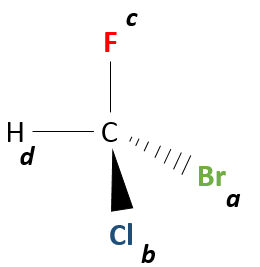
The first step is to give a priority to each group attached to a stereocentre.
Priority is given in regard with the mass of the atom directly bound to the stereocentre. Let’s name these groups a,b,c and d by decreased priority (A priors to B, B priors to C, C priors to D). If two atoms have the same weight (two carbon are bound to the stereocentre for example), we look at the atoms they are wearing and, again, the priority goes to the carbon wearing the atom of larger atomic weight. If a methyl and an ethyl were bound to the stereocentre, the ethyl has the priority. Both groups are bound to the stereocentre by a carbon atom. We look now at the atoms on these carbons. The methyl has 3H and ethyl has 2H and one C. As C is heavier than H, the ethyl has the priority on the methyl.
Remember that it is the weight of the bound atom that matters, not the weight of the complete group. A –OH groups has indeed the priority on an ethyl group because O (atomic weight=16) is larger than C (12) even if the groups weight respectively 17 and 29 units of atomic mass. Note that we are talking about the mass of the elements. Isotopes can thus create stereocentres in molecules.
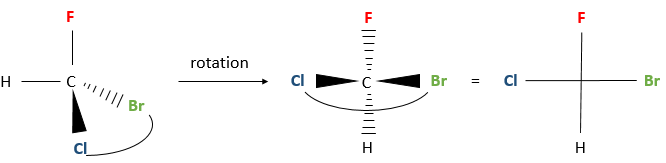
Next, we look at the molecule as if the group with the lowest priority (D) was behind the carbon. Generally the group with the smallest priority is a Hydrogen atom. This atom (or group) is not represented in the rest of the method.
So, looking at the stereocentre this way, we only see 3 liaisons connecting the stereocentre with the three groups of highest priority (A, B, C).
Now, we want to determine in which sense to rotate to go from the highest priority (A) to the lowest (C), passing by B. It may be helpful to place A in top of the representation.
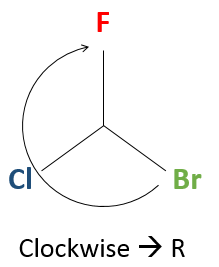
If we must go clockwise, it is the enantiomer R. If it is counterclockwise, we are in presence of the enantiomer S.
A second method exist, giving the same results, using the Fisher projection.
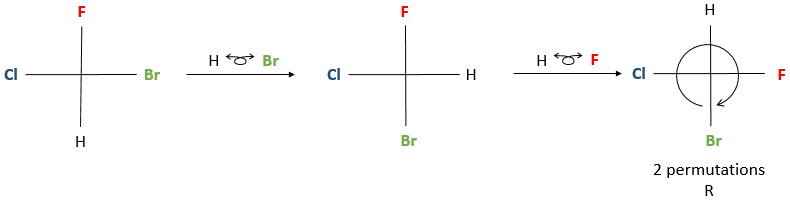
Instead of placing a group behind the stereocentre, we put two groups horizontally and two group vertically, still by a rotation of the stereocentre. The horizontally oriented groups are pointing toward the reader. The other two groups that are on the vertical axis are pointing opposite to the reader.
The rotation of the stereocentre is done by “grabbing” a pair or substituents and placing them in front of the molecule. Be careful to place the horizontal groups in the direction of the reader and the vertical ones in the opposite direction. Otherwise, a R enantiomer becomes S and vice versa.
Once the rotation is done, to determine the correct configuration, the group of lowest priority has to be placed on the 12 o’clock position of the Fisher representation. Then, the configuration is determined the same way as for the Newman projection. If the lowest priority group was not in the top position after the rotation, don’t worry, we can perform permutations between near substituents. Performing one permutation changes the conformation of the enantiomer from R to S and vice versa. Performing two let the enantiomer identical.
If an even number of permutations (including zero) were done to put the lowest priority group in the 12 o’clock position, we can determine directly the configuration of the enantiomer. If an odd number of permutation was done, then you have two choices: either you determine the present configuration, knowing that the correct configuration is the other one, or you do one additional permutation and then determine the configuration.
Diastereoisomers
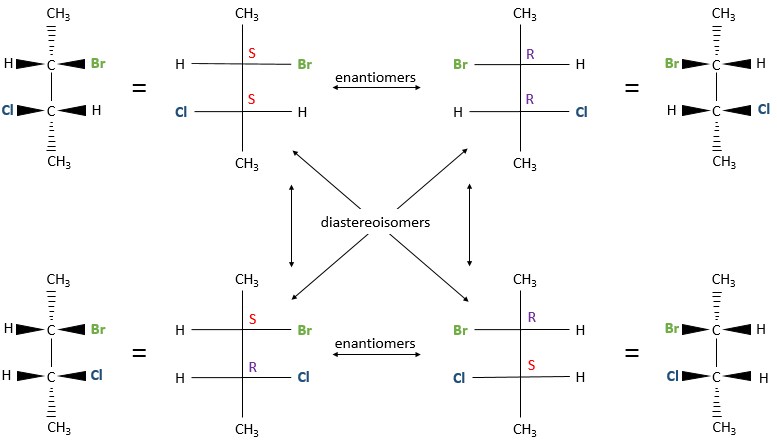
Several stereocentres may be present on a single molecule. The configuration of each stereocentre (R or S) is determined independently. If two stereocentres are on one molecule, several configurations are possible: RR, SS, RS and SR.
For example, 2-Bromo-3-chlorobutane has 4 stereoisomers.
All those conformations are not mirror image of each other. Some stereoisomers are enantiomers but some are not. Stereoisomers that are not the mirror image of each other are called diastereoisomer.
Exercises:
- Draw the skeleton structures of the isomers for C8H18. How many isomers did you find?
- Name the following molecules:
3. Draw the following molecule:
- 3,6,10-trimethyldodecane
- 5-ethyl-2-methyloctane
- 2,6-dimethyl-4,5-dipropylnonane
- 4-(1-methylethyl)heptane
- Is this name correct? If not, correct it.
- 2,5-dimethyl-4,6-dipropylnonane
- 3-ethyl-7-methyloctane
- 2,5-dimethyl-4-ethyldecane
- 4-(1-methylethyl)-5-propyldecane
- Draw the Newman projections of the C-C liaisons of this molecule and write if they are eclipsed or staggered
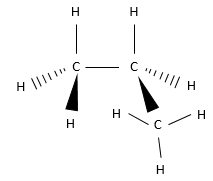
6. Chiral or achiral? If chiral, indicate if they are R or S.
Answers
1. There are 23 isomers of constitution for C8H18. A good way to find them all is to start from the longest main chain and decrease its length step by step.
2. Names:
2.1 n-hexane
2.2 Neopentane or 2,2-dimethylpropane
2.3 4-ethyl-7-methyldecane. It is not 4-methyl-7-ethyldecane because substituents are placed in alphabetical order if at the same distance from one extremity of the chain
2.4 6-propyl-3-methyldecane (main chain from top to bottom right)
2.5 5-(1-methylpropyl)decane
2.6 4-ethyl-4-methyloctane
3. Names and sketch:
3.1. 3,6,10-trimethyldodecane
3.2. 5-ethyl-2-methyloctane
3.3. 2,6-dimethyl-4,5-dipropylnonane
3.4. 4-(1-methylethyl)heptane
4. Correct or not?
- 2,5-dimethyl-4,6-dipropylnonane: correct
- 3-ethyl-7-methyloctane: incorrect: the methyl substituent is closer of an extremity than the ethyl. The correct name is 6-ethyl-2-methyloctane
- 2,5-dimethyl-4-ethyldecane: incorrect: substituents have to be named in the alphabetical order. The correct name is 4-ethyl-2,5-dimethyldecane
- 4-(1-methylethyl)-5-propyldecane: correct
5. Newman projections going from left to right
6. Chirality:
6.1 Chiral: R
6.2 Achiral
6.3 Chiral: S
6.4 Achiral
6.5 Chiral: S
Chapter 4 : Neutralization and titration
Neutralization
A neutralization reaction is the reaction occurring between an acid and a base forming a salt and water.
Technically, the neutralisation is not a one step reaction in the sense that all the actions are not done simultaneously but step by step. The fist step is the dissociation of the acid and base from their conjugate species. The second step is the formation of the salt from the conjugate species and of water from H3O+ and OH–. For example, NaOH is neutralised by HCl to form NaCl (cooking salt) and water.
The first equation is indeed the addition of the 4 equations underneath. If a compound is found at both sides of the equation, as the ionic species, we don’t write them in the mean equation. Note that all the molecules of Na+ and Cl–
are not reacting to give NaCl. There is here an equilibrium between the species in solution and the salt precipitating. This equilibrium is to be seen in the section of Dissolution.
The neutralization point is reached when the quantity of acid and base put in solution are equal. All the reactants are then consumed and for this reaction the pH is neutral, i.e. pH=7.
Titration of strong acids/bases
Titration is a method used to determine the concentration of a compound through its neutralisation. For example, the concentration of a solution of HCl can be determined by the addition of a solution of NaOH. The concentration of the titrating solution is known. As we are in presence of a strong acid (HCl) and a strong base (NaOH), both of them completely dissociate in solution. The concentration of proton is initially equivalent to the concentration of Cl–. In the other solution, the concentration of OH– and of Na+ is equivalent of the known concentration of NaOH.
To obtain a neutral pH, the number of protons na (in moles) has to be equal to the number of OH–, nb (in moles). In other words, neutralisation, or equivalence, is reached when
The number of moles of a compound in a solution is simply the concentration of this species multiplied by the volume of the solution:
From the two previous relations, we can find the initial concentration of acid that we wanted to determine:
As an example, if 20ml of NaOH 0.01M was required to neutralise a volume of 10ml of the HCl solution, [HCl]=0.02M.
In laboratory, titrations are performed as follow:
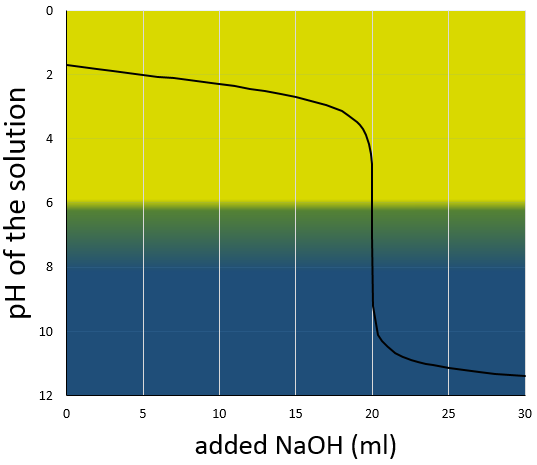
A flask containing a given volume of the unknown solution is placed on a magnetic mixer. The magnetic chip is placed in the solution to mix it continuously during the experiment. To be able to observe the neutralisation process, two droplets of pH indicator are added to the solution. Several indicators can be used to observe the passage through pH=7. Here, we will use bromothymol blue the color of which changes from yellow (pH<6.0) to blue (pH>7.6). At pH=7, the color is green. Considering the previous example, our solution is thus yellow. We can already say that the solution is acidic and that its pH is smaller than 6.
The neutralising solution, NaOH 0.01M in our case, fills a burette placed a few centimetres above the other solution. There is no need to add color indicator in this solution. Manipulating carefully the burette, NaOH is slowly added to the acid solution. One can read the consumed volume of base on graduations of the burette. When pH approaches 6, one can see the base droplets turning blue while mixing in the solution. Not knowing at all the concentration of the acid, it is convenient to perform a fast experiment to determine an approximate volume for the neutralisation and to perform a second experiment, only going slowly at the approach of this volume. Typically, the color will change from yellow to green or directly to blue at the fall of one droplet. The precision of this experiment is thus limited to the precision of the burette. Generally, the volume of a droplet is the half of a graduation.
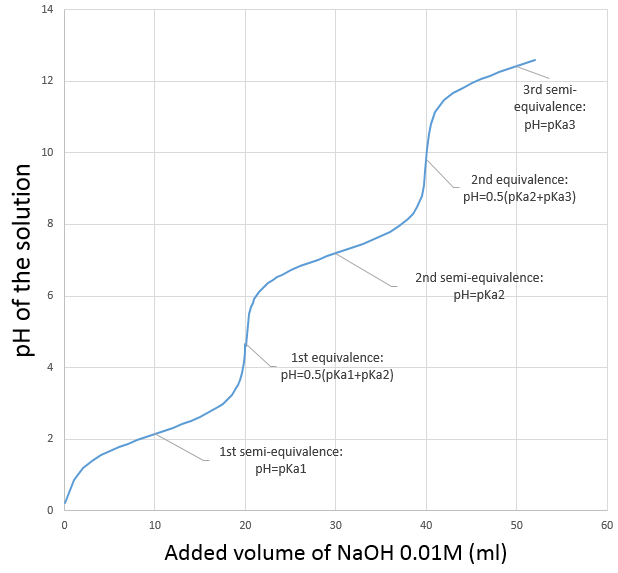
The titration curve of this example is shown next.
Remember that the pH is the logarithm of the concentration of protons while the addition of the base is linear, and that the volume of solution increases with the addition of the base (don’t forget that point when choosing the volume of the flask). As we can see, the pH variation is mainly focused at the vicinity of the neutralisation point. At 20ml, pH=7 but at 19.95ml (one less droplet approximately, for a 50ml burette), pH=4.78 and at 20.05ml, pH=9.22.
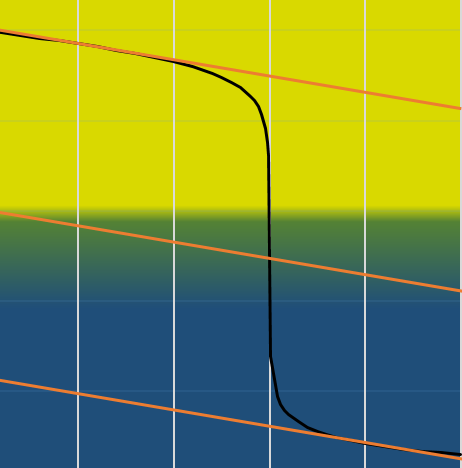
We can also find the equivalence point by tracing the tangents of the curve in the acid region and in the base region. Those two tangents are parallel. When we trace the line equidistant to those two line, it meets the equivalence point.
Titration of weak acids/bases
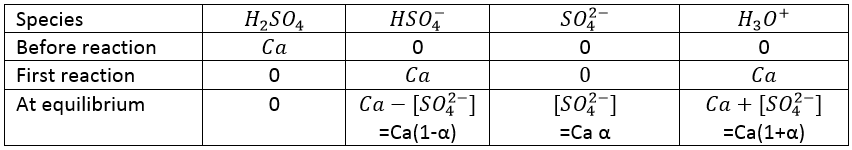
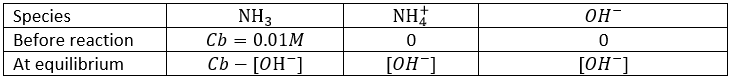
The titration of a weak acid is done using a strong base and follows the same principle. Lets perform the titration of the acetic acid. The reaction is
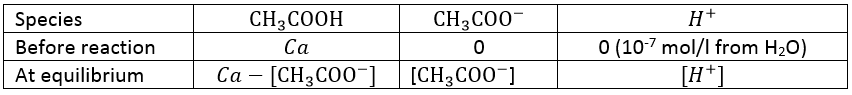
This reaction is complete. Before the titration, the pH of the acetic acid solution is simply given by the relation for a weak acid saw in the previous section
As for the strong acids, the equivalence is reached when
However, the pH at the equivalence is not neutral but basic. Indeed, the conjugate species of a strong base/acid is inert but the conjugate species of a weak acid/base is itself a weak base/acid: pKa + pKb = 14. All the acetic acid and NaOH are consumed but acetate has been produced and it is a weak base.
Before the equivalence, the pH depends on the quantity of weak acid and of its conjugate base:
This melange between a weak acid and its conjugate base is called a buffer solution because the addition of a strong base or acid does not modify sensibly the pH of the solution. Buffer solutions are very important for living species to resist to sudden variations of the environment. An example of buffer solution is our stomach. No matter what we eat or drink, its pH is unaffected (approximatively) so that the job can be done. In our whole body, enzymes are effective in a given area of pH and to keep them working, the pH has to be regulated through buffer solutions.
The semi-equivalence is the point when there is as much CH3COOH that CH3COO–(Ca=Cb). At this point, pH=pKa. To reach the semi-equivalence point, the added volume of base is the half of the volume to obtain the equivalence.
At the equivalence, pH is given by the amount of acetate in the solution (formula for a weak base). This amount is equal to the one of NaOH added to the solution.
After the equivalence point, pH is given by the quantity of NaOH in the solution.
Titration of a polyacid
Considering a polyacid HnA (a concrete example will be given later) with different enough pKa’s, the neutralizations of the different forms of the acid are successive: The OH– will first neutralize the protons released by HnA and then the protons released by Hn-1A–, etc.
The initial pH of the solution is the pH of HnA. The concentrations of the subsequent acids are negligible. HnA may be a strong acid or a weak acid.
At the first equivalence point, Hn-1A– is the main species in solution. It is an amphoteric species, i.e. it can accept or donate protons. The pH is thus
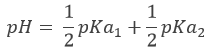
At the next semi-equivalence point, [Hn-1A–]=[ Hn-2A2-] and we are in a buffer solution. Remember that the pH in buffer solutions is
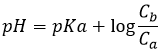
The pH is thus pH=pKa2. Note that if the initial acid is a weak acid, the same is true for the first semi-equivalence point, i.e. pH=pKa1.
It is interesting to note that for those specific values, pH does not depend on concentrations.
Let’s take the case of H3PO4 as an example. Its pKa’s are very different from each other
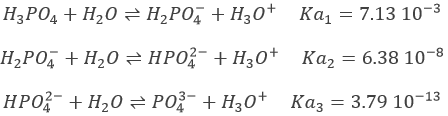
The neutralisations are successive and we can thus find the specific points (semi-equivalences and equivalences) determined above.
H3PO4 is a weak acid. The initial pH of the solution is thus
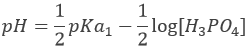
Before the equivalence, H3PO4 and H2PO4– are in solution. This buffer solution has a pH of
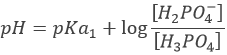
With pH=pKa1 when [H3PO4]=[H2PO4–], at the semi-equivalence.
At the first equivalence, H2PO4– is the main species in solution. It is an amphoteric species and the pH is thus
After the first equivalence, H2PO4– and HPO42- are in solution. This is again a buffer solution.
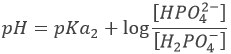
With pH=pKa2 when [H2PO4–]=[HPO42-], i.e. at the second semi-equivalence. At the second equivalence point, HPO42- is the main species in solution and is amphoteric.
After this equivalence, the solution is again a buffer solution.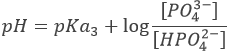
With pH=pKa3 when [HPO42-]=[PO43-], i.e. at the third semi-equivalence.
At the third equivalence, PO43- is the main species in solution. This is not an amphoteric species but a weak base. The pH should be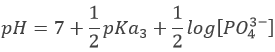
However, Ka3 is very close to Kw. Protons released by water are in competition with protons from HPO42- and the prediction does not stand anymore. To calculate the pH, we need to go back to the full composition of the solution and then solve the equation. This will not be done here.
Exercices
- What is the color of bromothymol blue in a 20ml solution of NaOH 0.005M? If we add 10ml, 20ml or 30ml of HCl 0.005M?
- What is the color of bromothymol blue in a 20ml solution of H3PO4 0.1M? If we add 10ml, 20ml or 30ml of NaOH 0.1M?
Answers
- 0ml: blue (pH=11.7), 10ml: blue (pH=11.22), 20ml: green (pH=7), 30ml: jellow (pH=3).
- 0ml: jellow (pH=1.57), 10ml: jellow (pH=pKa1=2.147), 20ml: jellow (pH=4.67), 30ml: green (pH=pKa2=7.2).
Chapter 3 : Strength of acids and bases
The general definition of an acid is thus a compound releasing protons. However, all the acids don’t have the same strength or acidity. We can define two types of acids and bases: Strong acids and bases, and weak acids and bases. For more simplicity, we will focus on acids in this lesson but the principle is identical for bases.
Strong acids
Strong acids totally dissociate in solution. It means that any single molecule of acid put in water will free a proton and acidify the solution. For example, HCl is a strong acid.
If one mole of HCl is put in water, all the HCl dissociates and in solution we can only find H2O, one mole of Cl– and one mole of H3O+. For this kind of reaction, the arrow separating reactants and products is a simple arrow going from left to right as the reaction is only going in one way. The potential Hydrogen, or pH, can thus simply be found with the quantity of HCl put in solution. pH=-log [H3O+] and as the reaction is complete, the quantity of H3O+ in solution is equal to the quantity of HCl put in solution. The concentration of protons is thus equal to the concentration of HCl in solution before the reaction [H3O+]=[HCl]0.
For example, if 0.1 mole of HCl is put in water to obtain a total volume of 1l, [H3O+]=0.1mol/l and pH=1. In lab, in general the acid is already in solution with a large concentration (6M for example) and has to be diluted to the desired concentration for the experiment. Remember that precautions are to be taken when you manipulate acids and bases, especially with concentrated ones. Use a pear to pipette them and not your mouth. Another “holy” rule is that “One does not baptise an acid”, meaning that to dilute an acid, add the acid into water and not water into the acid. The reason is that the dilution of an acid is highly exothermic and droplets of acid may be ejected out of the recipient.
The effect of a dilution on the pH is simple. If a solution of pH=2 ([H3O+]=0.01mol/l) is diluted 10 times, the pH increases by one (as it is a logarithmic scale) and pH=3 ([H3O+]=0.001mol/l), etc. For bases, a dilution decreases the pH of the solution towards the neutrality (pH=7). It is indeed the concentration of OH– which is affected in this case. As pH=-log[H3O+]=14+log[OH–].
Furthermore, a large dilution of an acid won’t lead to a basic solution. The dilution by 100 of a solution of pH=6 does not give a solution of pH=8 but approximately pH=7. In this case the water is the mean species defining the pH. The concentration of the protons coming from the acid becomes negligible with regard to the concentration of protons freed by the water.
To be considered a strong acid, the dissociation constant of the acid has to be large enough to proton all the H2O molecules of the solution into H3O+. Formally, strong acids have a pKa<-1.74. Lets explain that. We have seen that water has a dissociation constant of Kw=10-14mol2l-2. The dissociation constant of an acid is noted Ka. The same way as pH is –log of the proton concentration, pKa=-log Ka. For example HBr has a pKa of -8.7. The limit of pKa<-1.74 is simply the concentration of the water:
In 1l of water there is 1kg of H2O. The molar mass of H2O being equal to 18.01528g, the concentration of pure water is [H2O]=55.5084. –log of this concentration is 1.74.
To resume, to be able to proton all the molecules of water of the solution, which is the condition to be considered a strong acid, the acid has to have a pKa<-1.74. Some acids widely used are usually considered as strong acids but don’t answer to this condition but are between 0>pKa>-1.74 because they fully dissolve in diluted solution. These are the almost strong acids.
Among strong acids, we can find hydrochloric acid (HCl, almost strong acid), sulphuric acid (H2SO4), nitric acid (HNO3, almost strong acid), hydroiodic acid (HI), percloric acid (HClO4), hydrobromic acid (HBr) and many other.
Examples of strong bases: Sodium hydroxide (NaOH), Potassium hydroxide (KOH), Calcium hydroxide (Ca(OH)2),…
The conjugate base of strong acids are very weak bases and are inert as a base. Indeed, the basicity of the conjugate base of an acid (and inversely) is related to the Ka of the acid. The relation is Ka.Kb=Kw=10-14. Imagine for a second that the conjugate base reacts with water. If we add the reactions of the acid and of its conjugate base, we obtain the autoprotolyse of water:
HCl has a Ka=103 and the Kb of Cl– is thus Kb=10-17.
Weak acids
The acids do not all completely dissolve in water. Acids with pKa>0 are considered as weak acids. Because all of the molecules of acid do not dissolve, there is an equilibrium between the dissociated and the undissociated forms of the acid.
The equilibrium is represented by the two arrows between reactants and products.
An example of weak acid is the acetic acid (CH3COOH).
In solution, there is thus a melange of those 4 molecules. The pH is still determined by the amount of protons in solution. How do we find this quantity in the case of weak acids?
The constant of reaction of this reaction is
Lets take a look at the concentration of the species before and after the reaction
One part of the initial concentration of the acid (Ca) has reacted. The quantity of protons and of the conjugate base (CH3COO–) produced after the reaction are equal.
Generally, Ca>>[H+] (be carefull with this approximation for diluted solutions), leading to the following relation.
The pH of the solution can thus be found from the initial concentration of the acid put in solution and from its Ka:
For bases, the relation is similar:
Those relation can also be written as
From those equations, one can directly see why this kind of acid is weak with regard to strong acids: To obtain an increase of the pH of 1, a strong acid is diluted by 10 while a weak acid has to be diluted by 100.
Contrarily to strong acids, the conjugate bases of weak acids are active as a base in the solution. For example, the conjugate base of a weak acid with Ka=10-4 has a Kb=10-10.
Factors influencing the acidity
Electronegativity: The electronegativity refers to the ability of atoms to keep its electrons and the electrons of the bonds he shares near its nucleus. For two bonded atoms of same electronegativity, electrons composing the bond are not static but they spend an equal time at each end of the bond (statistically). For two bonded atoms of different electronegativity, electrons spend more time in the vicinity of the atom of larger electronegativity. This generates a separation of charges, a dipole with a partial negative charge (noted δ–) on the electronegative element and a partial positive charge (noted δ+) on the electropositive element. In acids, H possesses a partial positive charge depending on the electronegativity of the atom he is bonded to.
This partial positive charge stimulates the dissociation of H and thus increases the acidity of the molecule. Taking a look to the Mendeleev table, moving from left to right across a row on the periodic table elements become more electronegative (excluding the noble gases), and the strength of the acid formed by the element and hydrogen atoms increases accordingly.
Electronegative elements that are not directly bonded to the hydrogen can also pull electrons from the hydrogen. The effect is way smaller but should not be neglected.
Radius: Larger atoms have their bonding electrons further from the nucleus than small atoms. Because of this distance, these electrons are less energetic: there is a smaller interaction with the nucleus and the charge of the nucleus is partially shielded by the electrons of the inner layers. As a consequence, the bond is more easily broken to release a proton if the atom wearing the Hydrogen is large. The sequence of acidity for the halogenous acids shows it clearly. Hydrofluorous acid HF is a weak acid (pKa=3.2) and is less acid than HCl, HBr or HI even if its electronegativity is larger than theirs because it is much smaller (by a factor 2 to 3). The bond between the fluorine and the hydrogen is thus stronger because electrons are close to the nucleus. HI is the largest of the sequence and is also the most acidic halogenous acid with a pKa of -9.3>pKaHBr (-8.7)>pKaHCl (-6.3))>> pKaHF (3.2). Moving down a column on the Mendeleev table, the size of the elements increases and become less electronegative. The size effect tends to dominate the variation of electronegativity and the acidity increases of compounds wearing Hydrogens atoms.
Polyacids
Earlier, we mentioned the sulphuric acid, H2SO4. This acid has two protons available.
H2SO4 is a strong acid (pKa1=-3). When sulphuric acid is put in solution, a first proton is freed and there should be no remaining H2SO4 in solution. On the other hand, HSO4– is a weak acid (pKa2=1.9) and it will not totally dissociate in water. To determine the pH, we can proceed as we did for the weak acid:
With simple math, one can see that [HSO4–]= 2Ca- [H3O+] and we can write
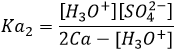
From that point, we obtain a second degree equation
![]()
That has now to be solved to obtain
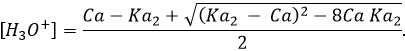
The pH can still be calculated from the constant of dissociation and from the initial concentration of acid put in solution even if the solution is a bit more difficult.
Amphoteric species
Amphoteric species are species who show both acidic and basic characteristics. HCO3– is an example of amphoteric species. As for the sulphuric acid, H2CO3 is a polyacid. However the carbonic acid is a weak acid and there is thus an equilibrium involving HCO3– as the conjugate base of H2CO3.
Water is also an amphoteric species as it can free or accept a proton.
There is a particular pH at which the amphoteric species has the same effect as a base than as an acid. This pH is called the isoelectric pH or pI. For H2O, the isoelectric pH is 7 but it can be determined from the value of Ka:
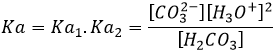
Considering that [H2CO3]=[ CO32-] at this pH,
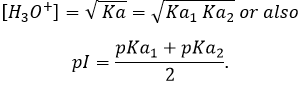
Amino acid is another amphoteric species. Such kind of molecule wears an acid group and a basic group. Fig.1 shows the structure of an amino acid in its acidic (left), neutral (middle) and basic form (right). The acid group of the amino acid is the COOH group. Its hydrogen atom can be released to obtain a negative charge on the oxygen. This charge is stabilised by resonance by the COO– group. The amine group on the left of the molecule has the role of the base. The azote possess a pair of electrons available to accept a proton.
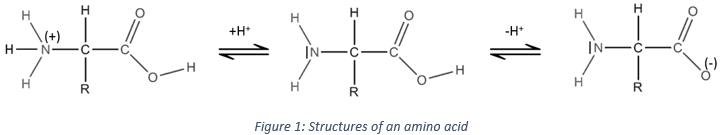
This amphoteric property of the amino acid is used experimentally to separate amino acids between them. Because of their particular structure (R varies depending on the amino acid), each amino acid has a different isoelectric pH. The molecules are placed on a gel containing a gradient of pH, inside an electric field. As long as the amino acid is not in its neutral form, it is attracted by an electrode placed at an extremity of the gel. Each amino acid will thus stop to move at a different place on the gel and can be separated. For example Alanine (R=CH3) has a pI=6 while pI=5.48 for the phenylalanine (R=CH2C6H5).
Exercices
You can find here a few exercices to apply the theory explained in this section and eventually sections related to its subject. Most of the questions should be simple to answer but some may require a calculator, or be thougher. Answers are given underneath.
- What is the pH of a solution of HCl 0.5M?
- How do I procede experimentally to obtain 100ml of HCl 0.05M?
- What is the pH of this solution?
- If I put a droplet of this solution on a bit of pH paper, what color does the paper take?
- Is HClO (Cl-O-H) an acid or a base?
- What is the pH of a solution of HClO 0.025M (pKa=7.497)?
- If this solution is diluted by 10, what is its pH? and if diluted by another 10?
- What is the pH of a solution of NaOH 0.01M? of NH3 0.01M (Kb=1.8×10−5)? At the equilibrium, how much NH3 remains in solution?
Answers:
- pH=0.3: HCl is a strong acid and completely dissociates in solution. pH=-log[H3O+]=-log[HCl]=0.3
- Even if the acid is not very concentrated in this case, it still has a high pH and precautions have to be taken to manipulate such a solution. Here, we just need to do a dilution of the acid solution by 10. To obtain 100ml of diluted solution, we will need a pipette of 10ml and its pear, and a volumetric flask of 100ml. One does not baptise an acid. The flask is thus first filled with a volume water (50ml for example) and 10ml of the acid solution is added using the pipette and its pear. Avoid to use your mouth. Mix the solution and add water to the graduation. Mix one more time.
- pH=1.3: As the initial solution has been diluted by 10, pH increases by 1. No need to calculate here.
- Deep red
- HClO is an acid: it was fully explained in the previous section: in HClO (Cl-O-H), the electronegativity difference between Cl-O is larger than the one between O-H. The hypochlorous acid splits in ClO– and H+. χ–Cl=3.16, χ–H=2.2, χ–O=3.44
- pH=4.55. The hypochlorous acid is a weak acid. The pH formula is then
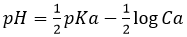
- pH=5.05 for a dilution by 10 and 5.55 for a dilution by 100.
- NaOH: pH=12, NH3: pH=10.63, [NH3]=0.009576M. We can here simply use the formula of weak bases for NH3 (remember that the pKa+pKb=pKe) but we will need the details for the third part of the question, so lets develop the problem:
Considering that Cb>>[OH–], and we will see that this approximation is correct, we find
So from the 0.01M of NH3 that were put in solution, only ~4% dissociate. The remaining concentration of NH3 is [NH3]=0.009576M. pH is found using pH=14+pOH=10.63.
Chapter 2 : acid-base reactions
In this module we will review one of the main types of reaction of chemistry. Reactions can indeed be classified in 3 major categories:
Acid-base reactions
Redox (reduction and oxidation) reactions
Solubility reactions (dissolution and precipitation)
The two last reaction types are seen in other sections of our lessons. We will here focus on acid-base reactions. The first step will be to introduce the definitions of acid and basic compounds and the notion of acidity. We will see next the strength of different acids and bases and explain how to follow experimentally the neutralisation of an acid by a base.
Definitions
A proton is a Hydrogen (H) atom that lost its electron (e-). Consequently, a proton consists only in one nucleus, which is positively charged. The proton is an ion: a charged molecule. We have here to consider that the nucleus of an atom is only a very small fraction of the volume of an atom (radius of GHAHR 1). Because of its small size, a proton can diffuse in everything and move through any material until its neutralization.
Several definitions were given to an acid and a base. Arrhenius proposed
An acid is a donator of protons
A base is a donator of OH-
This definition works well for several compounds, for example:
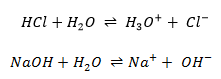
However, some basic compounds do not possess any OH group in them and can still neutralize acids. For example NH3 can react with H+ but cannot free any OH-. A tentative of explanation was to introduce NH4OH
![]()
but this compound does simply not exist.
Brønsted and Lowry proposed another theory:
An acid is a donator of protons
A base is an captor of protons
When a base reacts with an acid, they form respectively their conjugate acid and conjugate base :
![]()
Considering any acid HA, the equation can be written:
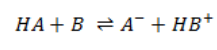
HA loses a proton to form its conjugate base A-. The base B receives the proton to form its conjugate acid HB+.
An interesting point of this theory is that the acidity of a compound depends on the reaction in which it intervenes. It allows to some compounds to be both an acid and a base. For example, H2O can donate or receive protons.
![]()
Such compound is called amphoteric. In water, there is thus both acid and base in water. However, when we drink or put a hand into water, we don’t feel those substances (note that the water that we usually use in our everyday tasks contains ions, modifying its taste and slightly its acidity). As explained earlier, an acid attacks deeply into materials. Bases on the other hand affect surfaces by removing their protons. So why is nothing happening? The reason why is because the three substance (H2O, H3O+ and OH-) are in equilibrium. The reaction just above goes in both directions, as it is shown by the double arrow. However, the reaction doesn’t go in both directions at the same speed. The equilibrium constant for the reaction going from left to right is :
![]()
For the other direction, the constant is K=1/Kw. That means that the equilibrium is highly oriented towards the left. It is not frequent that a molecule of H2O autoprotolyses and when it occurs, the inverse reaction is very fast.
From Kw, we can determine the concentration of protons (or H3O+) in water.
![]()
Because H3O+ and OH- are produced at the same rate, their concentration is equal: [H3O+]=[OH-]. Then
![]()
In pure water, the concentration of protons is thus 10-7M (M=mol/l) at any moment. If an acid is put into water, the amount of protons in solution will increase. Inversely, if a base is put in water, the amount of protons decreases. The acidity of a solution is thus measured by the concentration of protons in the solution. For more comfort, the scale, called potential Hydrogen or pH, is minus the logarithm of the concentration of protons:
![]()
and goes from 0 to 14 in aqueous solutions. At pH=0, the concentration of protons in solution is 1M. At pH=14, barely all the protons are removed from the solution by the base. The pH is not infinite because there is always a few protons remaining in the solution, due to the equilibrium. pH=7 is the neutral pH and is the pH of pure water. Most of the living species are adapted to this neutral pH. Some others have adapted to basic or acidic conditions to avoid predation or concurrence for resources.
We can also talk about pOH for bases with pOH=-log [OH-]. pOH is however generally not used. For basic aqueous solution, it is easier to refer at pH=14-pOH=14+log[OH-].
Lewis acids and bases :
The same year, Gilbert Newton Lewis proposed an alternative, and broader, definition for acids and bases: A Lewis base is defined as a compound that can donate an electron pair to a Lewis acid, a compound that can accept an electron pair. Considering the same notation as above,
LewisAB
The two dots in this notation represents the pair of electron that the Lewis base B and the conjugate base A- are carrying. The proton is a Lewis acid, accepting pairs of electrons. With such a definition, the acids are no more limited to substances carrying hydrogen atoms. For example BF3 is a Lewis acid as the bore can accept a pair of electron.
Dissociation of H and OH
It is to be noted that in some occasions, H is not dissociated as a proton. When the liaison between two atoms is broken, the pair of electrons remains with atom of greater electronegativity (χ-).
Examples:
H-Cl: χ-Cl=3.16 χ-H=2.2
In this case, and as expected, the pair of electrons remains on the chloride atom because its electronegativity is larger than the one of the Hydrogen.
Na-H: χ-Na=0.93 χ-H=2.2
The Sodium hydride is one of the few exceptions where the Hydrogen atom takes the pair of electrons. Indeed, its electronegativity is very low in comparison with H. This molecule splits in Na+ and H-.
If we look now to the liaison OH:
χ-O=3.44 χ-H=2.2
This group, typical in the basic compounds, can break to free a proton. A molecule carrying a OH group may then be acidic or basic depending on the atom connected to the oxygen.
In NaOH for example, the electronegativity of Na (χ-Na=0.93) is smaller than the one of the hydrogen (χ-H=2.2), by far. As a result, it is the bond between Na and O that breaks. As the O is already negatively charged, the O-H bond won’t split to give O2- and H+.
On the contrary, in HClO (Cl-O-H), the electronegativity difference between Cl-O is larger than the one between O-H. As a result, the hypochlorous acid splits in ClO- and H+.
To resume, the acidity of a substance depends on the reaction in which it takes part, and the presence of a H or a OH group in the substance does not mean that it is an acid or a base, and vice versa the fact that a substance is acidic or basic does not mean that this substance carries a H or OH group.
Measure of pH
Different methods exist to measure or to give an idea of the pH of a solution.
pH indicator
When a few droplets of pH indicator are added to a solution, the pH indicator gives the solution a colour depending on the acidity of the solution. Into a given area of pH the solution will be of a certain colour while the colour is different into another area of pH. Those area are not specifically 0-7 and 7-14 and depend on the pH indicator used. The change of colour is due to interactions between protons and the molecules of the pH indicator.
For example, bromocresol green is yellow in its acidic form and blue in its basic form. There is a transition area of pH for the bromocresol green between the pH from 3,8 to 5,4 where its colour is green, the colour of “its neutral form” (in fact it is a mix of the acidic and the basic forms). The structure of bromocresol green is shown is the Figure 1. The colour of the solution does not vary sensibly in the same area of pH but only at the limits between two area. It is explained by the fact that only a few drops are enough to obtain a visible colour. Moreover, as there are interactions between the pH indicator and the protons, the pH of the solution is affected by the presence of the pH indicator.
bromocresol
Figure 1: Structure of the bromocresol green in its acidic (left) and basic (middle and right) form. There are two resonance structures of the basic compound. Resonance will be seen in further chapters (organic chemistry)
Note that at pH=5.5 for example, this indicator is in its basic form even if the solution is acidic. pH indicators are thus useful to have an idea of the acidity of the solution. However, a lot of pH indicators exist and are easy to use.
pH paper
The pH paper is a paper containing several pH indicators.
dossiers-experimentale-analyse-article-Indicateurs_pH_Demirdjian-21
Initially jellow, its colour varies with the pH of the solution, from deep red for acids to deep blue for bases. Usually one droplet of the solution is dropped on the pH paper, giving it its colour. One can next compare the colour of the pH paper with a scale on the box of the pH paper to determine the pH of the solution.
pH meter
This device determines the concentration of protons in solution thanks to an electrode plunged in the solution. It is more accurate than the two other methods but may need calibration. Its functioning will be seen later.
Chapter 1: history of chemistry and chemical reactions
It is often useful to take a look on the history of something to understand it. That is how we will begin our lessons about chemistry. As far as we can go, chemistry started with the discovery of fire, which is basically the combustion of a reactant to obtain heat from it. Later, different metals were discovered, giving the names of the iron, copper and bronze eras.
However, we can’t talk about a scientific method at this moment yet. It is more about evolution. Evolution is a process to adapt best to our environment, most of the time through a trials and errors process. Processes which lead to a better adaptation were repeated while the others were not. Fire for example gave a huge improvement to the life of men for now obvious reasons but yet the process was not deeply understood.
Rationalisation is first seen with the Egyptians (fabrication of glass, beer and coloring), China (porcelain) and then with the Greeks. It is Leucippe and then Democrite who describe the matter as composed of small unbreakable particles, the atomos. Greeks also claimed that the world is composed of 4 main elements: earth, water, air and fire. We could now compare those to the three main phases: solid, liquid, gaz and the energy
The scientific method was developed during the XVI century. The method consists in 3 steps:
- Observation of a phenomenon: gives quantitative and qualitative information
- Hypothesis: tries to give possible explanations to the observed phenomenon
- Experiments: gathers new information on the phenomenon, confirm or not the theories developed in the previous step.
Before that, men described what they saw. From that point, men try to explain what they see through theories.
Stoichiometry and determination of the atomic masses
One of the fathers of the modern chemistry is Lavoisier. The statement he is known for is “Nothing is lost, nothing is created, everything is transformed” which meant that the total mass of the product of a reaction equals the total mass of the reactants. This statement is indeed true except for nuclear reactions during which a part of the mass is converted into energy.
Joseph Proust stated that a chemical compound always contains exactly the same proportion of elements by mass. For example, in pure water, the mass of hydrogen is always 1/9 of the mass of the sample while the oxygen makes up the 8/9 of the mass.
To complete this law, Dalton observed that during a reaction, the masses of the compounds which react together are always in a relation of simple integers. For example oxygen (O) and carbon (C) can react together in several ways
1g of C + 1.33g of O → 2.33g of CO
1g of C + 2.66g of O → 3.66g of CO2
to form carbon monoxide or carbon dioxide. The relationship between the mass of oxygen is 1 to 2. This is the basis of the stoichiometry. Bertholet protested against that law because one of his experiments gave opposite results. This experiment involved a solid of CuO wherein the ratio between Cu and O is neither constant nor a simple integer. The reason is that solids may have imperfections. Basically, these imperfections can be empty spaces or atoms replaced by others. This is why Bertholet obtained a formula of Cu1-xO instead of CuO.
Dalton established an atomic theory:
- All matter is made of atoms. Atoms are indivisible and indestructible.
- All atoms of a given element are identical in mass and properties. Atoms of different elements are different.
- Compounds are formed by a combination of two or more atoms. There is no formation of new atom (except nuclear reactions).
- A chemical reaction is a rearrangement of atoms.
The mass of each element has been determined. First works were performed by Cannizzaro, basing its experiment on a principle enounced by Avogadro: In normal conditions of temperature and pressure, identical volumes of gas have the same number of particles. Knowing the proportion of carbon there is in different gases, Cannizzaro determined its mass:
| Compound | Mass (g) | % of carbon | Mass of carbon (g) |
| Methane | 16 | 75 | 12 |
| Ethane | 30 | 80 | 24 |
| Propane | 40 | 82 | 36 |
The mass of C was determined this way. C has a mass of 12 atomic mass units (u). Consecutively, the mass of oxygen (16) has been determined from carbon dioxide (CO2). And so on. Initially, some errors occurred, typically because of elements with an even mass. For example, it was known that 2g of H react with 16g of O to form 18g of water. Considering the simplest relation, H has a mass of 1u (which is correct) but O would have a mass of 8u.
Moles and the Avogadro’s Number
The mole is one of the seven units of the International System of Units (SI Units): kilogram for mass, meter for length, second for time, Kelvin for temperature, ampere for electric current, candela for luminous intensity and mole for the amount of substance. The symbol for mole is mol.
Coming back to Avogadro, one of the most important numbers in chemistry, but almost never used is the Avogadro’s Number NA. As atoms are unbreakable, there are obviously several atoms in 12g of C. A mole expresses the number of atoms of carbon in 12g of carbon.
MC=NA.mC
This relation is true for any element i. Mi is the molar mass of i, i.e. the mass of one mole of the element i. Its units is g/mol (or g mol-1). mi is the mass of one atom of the element i. In the case of the carbon, MC=12g mol-1. mC being a mass, NA unit is mol-1. The value of NA was initially determined by Johann Josef Loschmidt who calculated the number of particles in a given volume of gas. The accuracy of the measure was perfectible and there are now experiments which give more accurate results than this method.
NA= 6.02214129(27)×1023 mol−1
Ourself and our environment is thus filled by an amazingly large number of atoms that interact together to form matter, air, liquids and most importantly life. The idea that molecules from the living could be crafted was not accepted before the XIX century. Friedrich Wöhler, a german chemist, can be considered as a pioneer of the organic chemistry. At this time, the hypothesis of vitalism was popular: any compound, to be living, needs a vital force given by God. Humans should not be able to synthesize, without this vital force, any organic compound. Wöhler proved that this theory was wrong by producing urea, accidentally, from inorganic substances. Even if urea is a waste of our body (when we pee), it is an organic compound and it should have been impossible to Wöhler to synthesize it without the intervention of the vital force from a living species. Friedrich Wöhler wanted to produce ammonium cyanate from potassium cyanate (KNCO) and ammonium chloride. However, the target product is unstable and acts only as an intermediary product, decomposing itself into urea.
Stoichiometry
The stoichiometry is the relation between the quantities of reactants and products during a chemical reaction.
A chemical reaction is written by an equation, placing the reactants on the left of an arrow and the products on the right of it. There can be several reactants that react together to form one single product. Each species is separated by a +.
In this reaction, hydrogen and oxygen are mixed to produce water (H2O). Hydrogen and oxygen are separated by a + at the left of the arrow because they are the reactants and the water is at the right of the arrow because it is the product of the reaction. Several products can be formed from one or more different reactants.
In this case, two products are generated by the chemical reaction: water and carbon dioxide. They are also separated by a + and are still at the right of the equation.
Now, the equations are not complete. We have to respect the law of conservation of mass (of Lavoisier): nothing is lost, nothing is created, everything is transformed. The quantities of an atom at the left and at the right of a chemical equation have to be identical. In the first equation, we wrote that one mole of H2 reacts with one mole of O2 to form one mole of H2O. The number of H is equal before and after the reaction (there are 2 of them in H2 and 2 in H2O) but one atom of oxygen would be lost. To obtain the correct equation, we put coefficients, called stoichiometric coefficients, before the species:
It is thus 2 moles of H2 that react with one mole oxygen to produce two moles of water. Here, the quantities of each atom is identical at each side of the arrow. This notation is also correct, as long as the numbers of atom are integers:
The second equation that we wrote was also incorrect:
The number of carbon C is correct, but the quantities of H and of O changed during the reaction. As there are 4 hydrogen at the left, we will put a coefficient 2 in front of the water to have 4 H at the right of the equation. Now, we have 4 oxygen’s in the products and only 2 in the reactants. To correct the equation, a coefficient 2 is put before the O2. The number of C is still correct and the equation is
There can be some variations to this notation. If a specific solvent is required for a reaction to happen, we indicate it above or under the arrow.
This reaction is the dissolution of salt (NaCl, also called table salt, the salt that we add on our food) into water. The elements of the salt are separated into the corresponding ions, i.e. charged species. The positively charged ions are called cations and the negatively charged species are called anions. If we need to heat the solution for the reaction to happen, we will also indicate it near the arrow by a Δ or a ΔT.
A reaction that requires heat to be made is an endothermic reaction. If the reaction generates heat, this reaction is said to be exothermic. Note that in the previous reaction, we indicated the states of the compounds between brackets. The g means that the species are gaseous. A s means that it is a solid, l stands for a liquid and aq stands for an aqueous solution. The heat generated by an exothermic reaction is written just as a product by Q or its exact value in kJ/mol if it is known. Some reactions produce light, also indicated as a product by hν, i.e. a photon of frequency ν.
The last point to talk about is that all the reactions are not complete. A complete reactions means that, if the reactants are put in stoichiometric proportions, all the reactants will be consumed during the reaction to form the products. If one reactant has more than the stoichiometric proportion, it is in excess and there will still be an amount of this reactant after the reaction, corresponding at the excess. During incomplete reactions, called reactions of equilibrium, all the reactants are not consumed even if they are put in stoichiometric proportions. There is an equilibrium between the quantities of reactants and of products of this reaction. Incomplete does not mean that the products are not fully made, but only that a part of them are generated. For example, the acetic acid is a low acid that does not completely dissolve in water.
As a result, we find the three species in solution: CH3COOH, CH3COO– and H+. Some of the reactants formed the products and some did not react. Note that the arrow in the chemical equation is different from the one of complete reactions. It is now two half arrows meaning that the reaction can go in both sides. For equilibrium reactions, we define a constant of equilibrium K such as
The [] means that we consider the concentrations of the species between the [] power their stoichiometric coefficient.
Exercises
1. Equilibrate those equations
2. If we put together 2g of Br2 and 1g of H2, how many moles of HBr can be produced? What is the mass of the excess of reactant?
3. Write the general equation for the combustion of the organic compounds CxHy and CxHyOz
Answers
1.
2. The reaction consumes 1 mole of each reactant to form 2 moles of HBr
As Br has the biggest molar mass, H2 will be in excess.
As a result, only 0.025mol of H2 is consumed and 0.05mol of HBr is produced by the reaction. The excess of H2 is 1.959molà1.975g of excess.
3. A combustion reaction is the reaction between one reactant and oxygen. From organic molecules, it generates CO2 and water.