Chapter 6 : the chemical composition of DNA
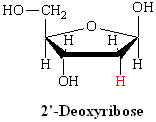
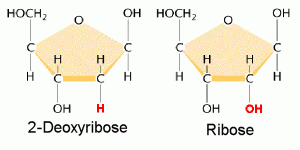
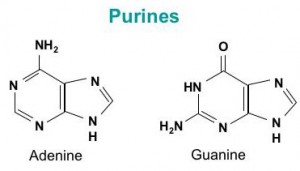
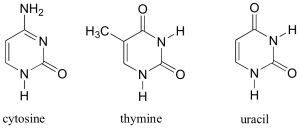
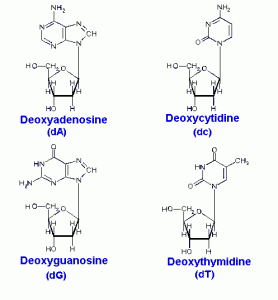
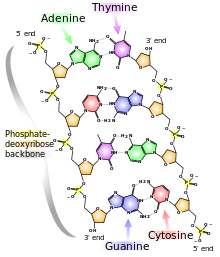
DNA is a polymer ( a large molecule that contains repeating units) composed of 2’ deoxyribose (a five-carbon sugar), phosphoric acid, and the four nitrogen containing bases denoted A, T, G and C. The chemical structures of the bases are shown below. Note that two of the bases have a double-ring structure, these are called purines. The other two bases have a single-ring structure, these are called pyrimidines.
The purine bases are adenine (A) and guanine (G).
The pyrimidine bases are thymine (T) and cytosine ©.
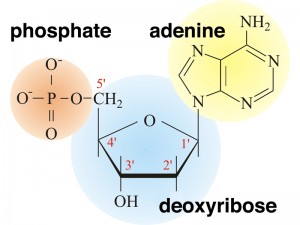
In DNA, each base is chemically linked to one molecule of the sugar deoxyribose, forming a compound called a nucleoside.
When a phosphate group is also attached to the sugar, the nucleoside becomes a nucleotide .
Thus a nucleotide is a nucleoside plus a phosphate. In the conventional numbering of the carbon atoms in the sugar,the carbon atom to which the base is attached is the 1’ carbon. (The atoms in the sugar are given primed numbers to distinguish them from atoms in the bases).
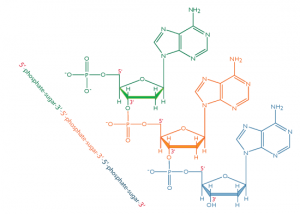
In nucleic acids, such as DNA and RNA, the nucleotides are joined to form a polynucleotide chain, in which the phosphate attached to the 5’ carbon of one sugar is linked to the hydroxyl group attached to the 3’ carbon of the next sugar in line . The chemical bonds by which the sugar components of adjacent nucleotides are linked through the phosphate groups are called phosphodiester bonds. The 5’-3’-5’-3’ orientation of these linkages continues throughout the chain are a 5’-phosphate (5’-P) group at one end and a 3’-hydroxyl (3’-OH) group at the other. The asymmetry of the ends of a DNA strand implies that each strand has a polarity determined by which end bears the 5’-phosphate and which end bears the 3’-hydroxyl.
Three years before Watson and Crick proposed their essentially correct three-dimensional structure of DNA as a double helix, Erwin Chargaff developed a chemical technique to measure the amount of each base present in DNA. As we describe his technique, we will let the molar concentration of any base be represented by the symbol for the base in square brackets; for example, [A] denotes the molar concentration of adenine. Chargaff used his technique to measure the [A], [T], [G], and [C] content of the DNA from a variety of sources. He found that the base composition of the DNA, defined as the present G+C, differs among species but is constant in all cells of an organism and within a species.
Chargaff also observed certain regular relationships among the molar concentrations of the different bases. These relationships are now called Chargaff’s rules:
The amount of adenine equals that of thymine: [A]=[T]
The amount of guanine equals that of cytosine: [G]=[C]
The amount of purine bases equals that of pyrimidine bases: [A]+[G]=[T]+[C]
Although the chemical basis of these observations was not known at the time, one of the appealing features of the Watson-Crick structure of paired complementary strands was that is explained Chargaff’s rules. Because A is always paired with T in double-stranded DNA, it must follow that [A]=[T]. Similarly, because G is paired with C, [G]=[C]. The third rule follows by addition of the other two: [A]+[G]=[T]+[C].
The Physical Structure of the Double Helix :
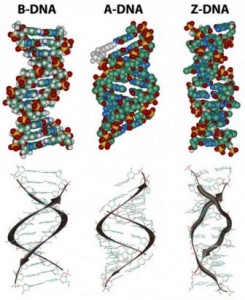
In the three-dimensional structure of the DNA molecule proposed in 1953 by Watson and Crick, the molecule consists of two polynucleotide chains twisted around one another to form a double-stranded helix in which adenine and thymine, and guanine and cytosine, are paired in opposite strands . In the standard structure, which is called the B form of DNA, each chain makes one complete turn every 34 A.
The helix is right-handed, which means that as you look down the barrel, each chain follows a clockwise path as it progresses. The bases are spaced at 3.4 A, so there are ten bases per helical turn in each strand and ten base pairs per turn of the double helix. Each base is paired to a complementary base in the other strand by hydrogen bonds, which provide the main force holding the strands together. (A hydrogen bond is a weak bond in which two negatively charged atoms share a hydrogen atom). The paired bases are planar, parallel to one another, and perpendicular to the long axis of the double helix. When discussing a DNA molecule, molecular biologists frequently refer to the individual strands as single strands or as single-stranded DNA; they refer to the double helix as double-stranded DNA or as a duplex molecule. The two grooves spiraling along outside of the double helix are not symmetrical; one groove, called the major groove, is larger than the other, which is called the minor groove. Proteins that interact with double-stranded DNA often have regions that make contact with the base pairs by fitting into the major groove, into the minor groove, or into both grooves.
The central feature of DNA structure is the pairing of complementary bases, A with T and G with C. The hydrogen bonds that form in the adenine-thymine base pair and in the guanine-cytosine pair are illustrated in Figure above Note that an A-T pair has two hydrogen bonds and that a G-C pair has three hydrogen bonds. This means that the hydrogen bonding between G and C is stronger in the sense that it requires more energy to break; for example, the amount of heat required to separate the paired strands in a DNA duplex increases with the percent of G + C. Because nothing restricts the sequence of bases in a single strand, any sequence could be present along one strand. This explains Chargaff’s observation that DNA from different organisms may have different base compositions. However, because the strands in duplex DNA are complementary, Chargaff’s rules of [A] = [T] and [G] = [C] are true whatever the base composition.
Each backbone in a double helix consists of deoxyribose sugars alternating with phosphate groups that link the 3’ carbon atom of one sugar to the 5’ carbon of the next in line. The two polynucleotide strands of the double helix are oriented in opposite directions in the sense that the bases that are paired are attached to sugars above and below the plane of pairing, respectively. offset because the phosphate linkages in the backbones run in opposite directions , and the strands are said to be antiparallel. This means that each terminus of the double helix possesses one 5’-P group (on one strand) and one 3’-OH group (on the other strand), as shown in Figure below :
The diagrams of the DNA duplexes are static and so some-what misleading. DNA is a dynamic molecule, constantly in motion. In some regions, the strands can separate briefly and then come together again in the same conformation or in a different one. Although the right-handed double helix is the standard form, DNA can form more than 20 slightly different variants of right-handed helices, and some regions can even form helices in which the strands twist to the left (called the Z form of DNA). If there are complementary stretches of nucleotides in the same strand, then a single strand, separated from its partner, can fold back upon itself like a hairpin. Even triple helices consisting of three strands can form in regions of DNA that contain suitable base sequences.
What a Genetic Material Needs That DNA Supplies :
Not every polymer would be useful as genetic material. However, DNA is admirably suited to a genetic function because it satisfies the three essential requirements of a genetic material. First, any genetic material must be able to be replicated accurately, so that the information it contains is precisely replicated and inherited by daughter cells added to the end of a growing protein molecule. In DNA, this is done by means of a genetic code in which groups of three bases specify amino acids. Because the four bases in a DNA molecule can be arranged in any sequence, and because the sequence can vary from one part of the molecule to another and from organism to organism, DNA can contain a great many unique regions, each of which can be a distinct gene. A long DNA chain can direct the synthesis of a variety of different protein molecules.
A genetic material must also be capable of undergoing occasional mutations in which the information it carries is altered. Furthermore, the mutant molecules must be capable of being replicated as faithfully as the parental molecule, so that mutations are heritable. Watson and Crick suggested that heritable mutations might be possible in DNA by rare mispairing of the bases, with the result that an incorrect nucleotide becomes incorporated into a replicating DNA strand.
Chapter 5 : stem cells
What is a stem cell?
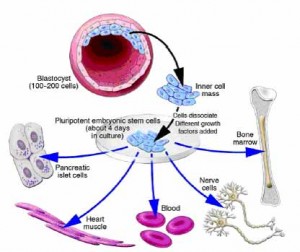
Ultimately, every cell in the human body can be traced back to a fertilized egg that came into existence from the union of egg and sperm. But the body is made up of over hundreds of different types of cells. All of these cell types come from a pool of stem cells in the early embryo. During early development, as well as later in life, various types of stem cells give rise to the specialized or differentiated cells that carry out the specific functions of the body, such as blood, muscle,bone and nerve cells.
Stem cells can regenerate themselves or produce specialized cell types. This property make stem cells appealing for scientists seeking to create medical treatments that replace lost or damaged cells.
TYPES OF STEM CELLS :
Stem cells are found in all of us, from the early stages of human development to the end of life. All stem cells may prove useful for medical research, but each of the different types has both promise and limitations. Embryonic stem cells, which can be derived from a very early stage in human development, have the potential to produce all of the body’s cell type. Adult stem cells, which are found in certain tissues in fully developed humans, from babies to adults, may be limited to producing only certain types of specialized cells. Recently, scientists have also identified stem cells in umbilical cord blood and the placenta that can give rise to the various types of blood cells.
Embryonic Stem Cells :
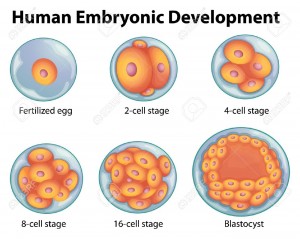
A blastocyst, is a pre-implantation embryo that develops 5 days after the fertilization of an egg by a sperm. It contains all the material necessary for the development of a complete human being. The blastocyst is a mostly hollow sphere. In its interior is the inner cell mass, which is composed of 30-34 cells that are referred to by scientists as pluripotent because they can differentiate into all of the cell types of the body.
In normal development, the blastocyst would implant in the wall of the uterus to become the embryo and continue developing into a mature organism. Its outer cells would begin to from the placenta and the inner cell mass would begin to differentiate into the progressively more specialized cell types of the body.
When the blastocyst is used for stem cell research, scientists remove the inner cell masse and place these cells in a culture dish with a nutrient-rich liquid where they give rise to embryonic stem cells. Embryonic stem cells seem to be more flexible than stem cells found in adults, because they have the potential to produce every cell type in the human body. They are also generally easier to collect, purify and maintain in the laboratory than adult stem cells. Scientists can induce embryonic stem cells to replicate themselves in an undifferentiated state for very long periods of time before stimulating them to create specialized cells. This means that just a few embryonic stem cells can build a large bank of stem cells to be used in experiments. However, such undifferentiated stem cells could not be used directly for tissue transplants because they can cause a type of tumor called a teratoma. To be used for therapies, embryonic stem cells would first need to be differentiated into specialized cell types.
Some find embryonic stem cell research to be morally objectionable, because when scientists remove the inner cell mass, the blastocyst no longer has the potential to become a fully developed human being.
Sources of Embryonic Stem Cells (In Vitro Fertilization):
The largest potential source of blastocysts for stem cell research is from in vitro fertilization (IVF) clinics. The process of IVF requires the retrieval of a woman’s eggs via a surgical procedure after undergoing an intensive regimen of “fertility drugs,” which stimulate her ovaries to produce multiple mature eggs. When IVF is used for reproductive purposes, doctors typically fertilize all of the donated eggs in order to maximize their chance of producing a viable blastocyst that can be implanted in the womb. Because not all the fertilized eggs are implanted, this has resulted in a large bank of “excess” blastocysts that are currently stored in freezers around the country. The blastocysts stored in IVF clinic could prove to be a major source of embryonic stem cells for use in medical research. However, because most of these blastocysts were created before the advent of stem cell research, most donors were not asked for their permission to use these left-over blastocysts for research.
The in vitro fertilization (IVF) technique could potentially also be used to produce blastocysts specifically for research purposes. This would facilitate the isolation of stem cells with specific genetic traits necessary for the study of particular diseases. For example, it may be possible to study the origins of an inherited disease like cystic fibrosis using stem cells made from egg and sperm donors who have this disease. The creation of stem cells specifically for research using IVF is, however, ethically problematic for some people because it involves intentionally creating a blastocyst that will never develop into a human being.
Nuclear Transfer:
The process called nuclear transfer offers another potential way to produce embryonic stem cells. In animals, nuclear transfer has been accomplished by inserting the nucleus of an already differentiated adult cell-for example, a skin cell-into a donated egg that has had its nucleus removed. This egg, which now contains the genetic material of the skin cell, is then stimulated to form a blastocyst from which embryonic stem cells can be derived. The stem cells that 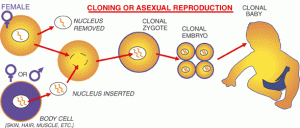 are created in this way are therefore copies or “clones” of the original adult cell because their nuclear DNA matches that of the adult cell.
are created in this way are therefore copies or “clones” of the original adult cell because their nuclear DNA matches that of the adult cell.
As of the summer of 2006, nuclear transfer has not been successful in the production of human embryonic stem cells, but progress in research suggests that scientists may be able to use this technique to develop human stem cells in the future.
Producing Embryonic Stem Cells Using Nuclear Transfer Is Not the Same as Reproductive Cloning
The use of nuclear transfer to develop disease-specific stem cells can be called research cloning, and the use of this technique for personalized tissue transplants is sometimes called therapeutic cloning. These terms must be carefully distinguished from reproductive cloning, in which the intent is to implant a cloned embryo in a female’s womb and allow it to develop fully into an individual. This was the technique by which Dolly the sheep was made and is now widely used for reproductive cloning in animals. In humans, however, reproductive cloning has been actively discouraged by most in the scientific community.
Scientists believe that if they are able to use nuclear transfer to derive human stem cells, it could allow them to study the development and progression of specific diseases by creating stem cells containing the genes responsible for certain disorder. In the future, scientists may also be able to create “personalized” stem cells that contain only the DNA of a specific patient. The embryonic stem cells created by nuclear transfer would be genetically matched to a person needing a transplant, making it far less likely that the patient’s body would reject the new cells than it would be with traditional tissue transplant procedures.
Although using nuclear transfer to produce stem cells is not the same as reproductive cloning, some are concerned about the potential misapplication of the technique for reproductive cloning purposes. Other ethical considerations include egg donation, which requires informed consent, and possible destruction of blastocysts.
Adult Stem Cells :
Adult stem cells are hidden deep within organs, surrounded by millions of ordinary cells, and may help replenish some of the body’s cells when needed. In fact, some adult stem cells are currently being used in therapies. They have been found in several organs that need a constant supply of cells, such as the blood, skin and lining of the gut, and have also been found in surprising places like the brain, which is not known to readily replenish its cells. Unlike embryonic stem cells, adult stem cells are already somewhat specialized. For example, blood stem cells normally only give rise to the many types of blood cells, and nerve stem cells can only make the various types of brain cells. Recent research however, suggests that some adult stem cells might be more flexible than previously thought, and may be made to produce a wider variety of cells types. For example, some experiments have suggested that blood stem cells isolated from adult mice may also be able to produce liver, muscle and skin cells, but these results are not yet proven and have not been demonstrated with human cells. Nevertheless, scientists are working on finding a way to stimulate adult stem cells, or even other types of adult cells, to be more versatile. If they succeed, it could provide another source of unspecialized stem cells.
Identifying Stem Cells :
As early as 1961, scientists knew that adult bone marrow contained cells that could make all of the blood cell types. But it wasn’t until 1988 that those stem cells were isolated as pure populations. Why did it take so long? The techniques for identifying stem cells have only recently been developed. Partly, this is because adult stem cells are, by their very nature, inconspicuous in shape, size and function. They also tend to hide deep in tissues and are present only in very low numbers, making their identification and isolation like finding a needle in a haystack.
How do scientists know when they have found a stem cell? Every cell displays an array of proteins on its surface; different cell types have different proteins. Scientists can use these surface proteins as “markers” that characterize individual cell types – a type of molecular ID. For example, using molecules that recognize and attach to specific surface proteins and that can fluoresce under certain wavelengths of light, scientists can visually tell the difference between a blood cell. Unfortunately, not all stem cells can now be identified in this manner because scientists have not yet identified markers for all stem cell types. Scientists also identify stem cells by observing their behavior in the laboratory: stem cells must be able to remain unspecialized and self-renew for long periods of time.
Scientists believe that there might be more types of adult stem cells than the handful that have already been identified, but finding them is a difficult process.
Culturing Cell Lines and Stimulating Stem Cells to Differentiate
Cell culture is a term that refers to the growth and maintenance of cells in a controlled environment out-side of an organism. A successful stem cell culture is one that keeps the cells healthy, dividing and unspecialized. The culturing of stem cells in the first step in establishing a stem cell line – a propagating collection of genetically identical cells. Cell lines are important because they provide a long-term supply of multiplying cells that can be shared among scientists for research and therapy development. The National Academies report Stem Cells and the Future of Regenerative Medicine (2001) described some of the challenges of maintaining cell lines: “Over time, all cell lines…change, typically accumulating harmful genetic mutations. There is no reason to expect stem cell lines to behave differently. While there is much that can be learned using existing stem cell lines…such concerns necessitate continued monitoring of these cells as well as the development of new stem cell lines in the future.”
Once they have established a stable stem cell line, scientists start the process of causing the stem cells to differentiate into specialized cell types. The cellular environment in which stem cells naturally reside provides scientists with clues about how to make them differentiate in a culture dish. For example, in the bone marrow, where blood stem cells reside, bone cells send physical and chemicals signals that tell the blood stem cells when to differentiate. Scientists are just beginning to understand these signals and have developed ways to mimic the natural processes in cell cultures. Usually, the technology involves adding certain proteins to the cell culture and, in some cases, introducing specific genes into the stem cells.
It will be essential that scientists are sure that stem cells have fully differentiated before they can use them for medical applications. If completely undifferentiated stem cells (such as embryonic stem cells) are implanted directly into an organism, they can cause a type of tumor called a teratoma, which scientists have observed in experiments using mice. Semi-specialized adult stem cells and differentiated cells derived from embryonic stem cells are unlikely to cause teratomas.
Alternatives to Using Embryos in Stem Cell Research
To address ethical concerns about the destruction of blastocysts, scientists are trying to find new ways of obtaining stem cells that behave like embryonic stem cells but that don’t require harming a blastocyst. As the science progresses, ethical issues surrounding these alternatives may also arise. Some possible alternatives include:
1) Cells collected from the morul, the developmental stage prior to the blastocyst. The morula, a solid ball of about 16-30 cells, seems able to sustain the loss of a few cells without developmental damage so that the remaining cells can continue to develop. Cell extraction from the morula is already being used in some clinics to screen for genetic disorders in embryos produced by in vitro fertilization. Researchers have recently shown that cells isolated from a mouse morula can give rise to embryonic stem cells while the remaining morula cells develop into a healthy mouse. However, this process may still be morally objectionable to some because of the chance of harm to the morula, and because the long-term effects of removing cells from a morula are not yet known.
2) The creation of embryonic stem cells through a process called altered nuclear transfer (ANT). In this variation of the nuclear transfer technique, scientists create a blastocyst create whose genetic material has been changed so that further development and implantation into the uterus is not possible. It aims to create embryo-like entities that are not truly embryos but that can be a source of pluripotent stem cells. ANT, so far only tested with mouse blastocysts, could allow the creation of embryonic stem cells without destroying a viable human blastocyst. Some who object to embryonic stem cell research support ANT because the resulting blastocyst could never develop into a full human being and therefore would not have the moral status of a human embryo. However, this procedure is objectionable to some because they believe that it involves the creation of an imperfect blastocyst that is designed to be destroyed.
3) Causing an adult cell to act like an embryonic stem cell. During development, as cells become more and more specialized, they gradually lose the ability to turn on the genes that allow embryonic stem cells to be so versatile. The silencing if these genes seems to be responsible for keeping specialized cells specialized and limiting the differentiation capacities of adult stem cells. By “reprogramming” adult stem cells so that they can turn on the genes that allow versatility, scientists hope to cause them to revert to a more flexible state. It is even possible that scientists could one day “reprogram” any cell, not only stem cells. However, research in this area is in the early stages and scientists may be many years away from making an adult cell as versatile as an embryonic stem cell.
Chapter 4 : specific features of meiosis
Meiosis has three unique features :
The mechanism of cell division varies in important details in different organism. This is particularly true of chromosomal separation mechanism, which differ substantially in protists and fungi from the process in plants and animals that we will describe here. Meiosis in a diploid organism consists of two rounds of division, mitosis of one. Although meiosis and mitosis have much in common, meiosis has three unique features: synapsis, homologous recombination, and reduction division.
Synapsis :
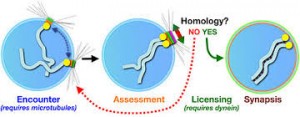
The first unique feature of meiosis happens early during the first nuclear division. Following chromosome replication, homologous chromosomes, or homologous pair all along their length. The process of forming these complexes of homologous chromosomes is called synapsis.
Homologous Recombination :
The second unique feature of meiosis is that genetic exchange occurs between the homologous chromosomes while they are thus physically joined. The exchange process that occurs between paired chromosomes is called crossing over. Chromosomes are then drawn together along the equatorial plane of the dividing cell; subsequently, homologues are pulled by microtubules toward opposite poles of the cell. When this process is complete, the cluster of chromosomes at each pole contains one of the two homologues of each chromosome. Each pole is haploid, containing half the number of chromosomes present in the original diploid cell. Sister chromatids do not separate from each other in the first nuclear division, so each homologue is still composed of two chromatids.
Reduction Division :
The third unique feature of meiosis is that the chromosomes do not replicate between the two nuclear divisions, so that at the end of meiosis, each cell contains only half the original complement of chromosomes. In most respects, the second meiotic division is identical to a normal mitotic division. However, because of the crossing over that occurred during the first division, the sister chromatids in meiosis 2 are not identical to each other.
Meiosis is a continuous process, but it is most easily studied when we divide it into arbitrary stages. The stages of meiosis are traditionally called meiosis 1 and meiosis 2. Like mitosis, catch stage is subdivided further into prophase, metaphase, anaphase and telophase. In meiosis, however, prophase 2 is more complex than in mitosis. In meiosis, homologous chromosomes become intimately associated and do not replicate between the two nuclear divisions.
The Red Queen Hypothesis. One evolutionary advantage of sex may be that it allow populations to “store” recessive alleles that are currently bad but have promise for reuse at some time in the future. Because populations are constrained by a changing physical and biological environment, selection is constantly acting against such alleles, but in sexual species can never get rid of those sheltered in heterozygotes. The evolution of most sexual species, most of the time, thus manages to keep pace with ever-changing physical and biological constraints. This “treadmill evolution” is sometimes called the “Red Queen hypothesis”, after the Queen of Hearts in Lewis Carroll’s Through the Looking Glass, who tells Alice, “Now, here, you see, it takes all the running you can do, to keep in the same place.”
Miller’s Ratchet. The geneticist Herman Miller pointed out in 1965 that asexual populations incorporate a kind of mutational ratchet mechanism – once harmful mutations arise, asexual populations have no way of eliminating them, and they accumulate over time, like turning a ratchet. Sexual populations, on the other hand, can employ recombination to generate individuals carrying fewer mutations, which selection can then favor. Sex may just be a way to keep the mutational load down.
The Evolutionary Consequences of Sex
While our knowledge of how sex evolved is sketchy, it is abundantly clear that sexual reproduction has an enormous impact on how species evolve today, because of its ability to rapidly generate new genetic combinations. Independent assortment, crossing over, and random fertilization each help generate genetic diversity.
Whatever the forces that led to sexual reproduction, its evolutionary consequences have been profound. No genetic process generates diversity more quickly; and, as you will see in later chapters, genetic diversity is the raw material of evolution, the fuel that drives it and determines its potential directions. In many cases, the pace of evolution appears to increase as the level of genetic diversity increases. Programs for selecting larger stature in domesticated animals such as cattle and sheep, for example, proceed rapidly at first, but then slow as the existing genetic combinations are exhausted; further progress must then await the generation of new gene combinations. Racehorse breeding provides a graphic example: thoroughbred racehorses are all descendants of a small initial number of individuals, and selection for speed has accomplished all it can with this limited amount of genetic variability – the winning times in major races ceased to improve decades ago.
Paradoxically, the evolutionary process is thus both revolutionary process is thus both revolutionary and conservative. It is revolutionary in that the pace of evolutionary change is quickened by genetic recombination, much of which results from sexual reproduction. It is conservative in that evolutionary change is not always favored by selection, which may instead preserve existing combinations of genes. These conservative pressures appear to be greatest in some asexually reproducing organism that do not move around freely and that live in especially demanding habitats. In vertebrates, on the other hand, the evolutionary premium appears to have been on versatility, and sexual reproduction is the predominant mode of reproduction by an overwhelming margin.
The close association between homologous chromosomes that occurs during meiosis may have evolved as mechanism to repair chromosomal damage, although several alternative mechanism have also been proposed.
The evolutionary origin of sex is a puzzle.
Why Sex?
Not all reproduction is sexual. In asexual reproduction, an individual inherits all of its chromosomes from a single parent and is, therefore, genetically identical to its parent. Bacterial cells reproduce asexually, undergoing binary fission to produce two daughter cells containing the same genetic information. Most protists reproduce asexually except under conditions of stress; then they switch to sexual reproduction. Among plants, asexual reproduction is common, and many other multicellular organism are also capable of reproducing asexually. In animals, asexual reproduction often involves the budding off of a localized mass of cell, which grows by mitosis to form a new individual.
Chapter 3 : meiosis
The sequence of events during meiosis involves two nuclear divisions ( meiosis I and meiosis II ).
Prophase 1
In prophase 1 of meiosis, the DNA coils tighter, and individual chromosomes first become visible under the light microscope as a matrix of fine threads. Because the DNA has already replicated before the onset of meiosis, each of these threads actually consist of two sister chromatids joined at their centromeres. In prophase 1, homologous chromosomes become closely associated in synapsis, exchange segments by crossing over, and then separate.
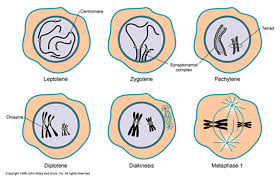
Prophase 1 is traditionally divided into five sequential stages: leptotene, zygotene, pachytene, diplotene, and diakinesis.
Leptotene: during which each chromosome becomes visible as two fine threads (chromatids)
Zygotene: A lattice of protein is laid down between the homologous chromosomes in the process of synapsis, forming a structure called a synaptonemal complex.
Pachytene: Pachytene begins when synapsis is complete (just after the synaptonemal complex forms and lasts for days. This complex, about 100 nm across, holds the two replicated chromosomes in precise register, keeping each gene directly across from its partner on the homologous chromosome. Within the synaptonemal complex, the DNA duplex unwind at certain sites, and single strands of DNA from base-pairs with complementary strands on the other homologue. The synaptonemal complex thus provides the structural framework that enables crossing over between the homologues chromosomes. As you will see, this has a key impact on how the homologues separate later in meiosis.
Diplotene: the fourth stage of the prophase I of meiosis, following pachytene, during which the paired chromosomes begin to seperate (the synaptonemal complex disassembles) into two pairs of chromatids.Diplotene is a period of intense cell growth. During this period the chromosomes decondense and become very active in transcription
Diakinesis: At the beginning of diakinesis, the transition into metaphase, transcription ceases and the chromosomes recondense.
Synapsis: During prophase, the ends of the chromatids attach to the nuclear envelope at specific sites. The sites the homologous attach to are adjacent, so that the members of each homologous pair of chromosomes are brought close together. They then line up side by side, apparently guided by heterochromatin sequences, in the process called synapsis.
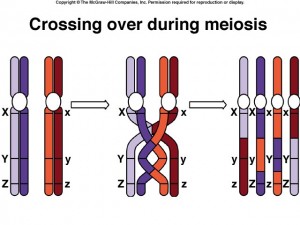
Crossing Over
Within the synaptonemal complex, recombination is thought to be carried out during pachytene by very large protein assemblies called recombination nodules. A nodule’s diameter is about 90 nm, spanning the central element of the synaptonemal complex. The details of the crossing over process are not well understood, but involve a complex series of events in which DNA segments are exchanged between nonsister or sister chromatids. In humans, an average of two or three such crossover events occur per chromosome pair.
When crossing over is complete, the synaptonemal complex breaks down, and the homologous chromosomes are released from the nuclear envelope and begin to move away from each other. At this point, there are four chromatids for each other. At this point, there are four chromatids for each type of chromosome (two homologous chromosomes, each of which consists of two sister chromatids). The four chromatids do not separate completely, however, because they are held together in two ways: (1) the two sister chromatids of each homologue, recently created by DNA replication, are held near by their common centromeres; and (2) the paired homologues are held together at the points where crossing over occurred within the synaptonemal complex.
Chiasma Formation
Evidence of crossing over can often be seen under the light microscope as an X-shaped structure known as a chiasma. The presence of a chiasma indicates that two chromatids (one from cach homologue) have exchanged parts. Like small rings moving down two strands of rope, the chiasmata move to the end of the chromosome arm as the homologous chromosomes separate.
Synapsis is the close pairing of homologous chromosomes that takes place early in prophase 1 of meiosis. Crossing over occurs between the paired DNA strands, creating the chromosomal configurations known as chiasmata. The two homologues are locked together by these exchanges and they do not disengage readily.
Metaphase 1
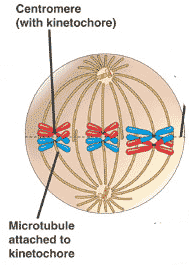
By metaphase 1, the second stage of meiosis 1, the nuclear envelope has dispersed and the microtubules form a spindle, just as in mitosis. During diakinesis of prophase 1, the chiasmata move down the paired chromosomes from their original points of crossing over, eventually reaching the ends of the chromosomes. At this point, they are called terminal chiasmata. Terminal chiasmata hold the homologous chromosomes together in metaphase 1, so that only one side of each centromere faces outward from the complex; the other side is turned inward toward the other homologue. Consequently, spindle microtubules are able to attach to kinetochore proteins only on the outside of each centromere, and the centromeres of the two homologues attach to microtubules originating from opposite poles. This one-sided attachment is in marked contrast to the attachment in mitosis, when kinetochores on both sides of a centromere bind to microtubules.
Each joined pair of homologues then lines up on the metaphase plate. The orientation of each pair on the spindle axis is random: either the maternal or the paternal homologue may orient toward a given pole.
Chiasmata play an important role in aligning the chromosomes on the metaphase plate.
Completing Meiosis
After the long duration of prophase and metaphase, which together make up 90% or more of the time meiosis 1 takes, meiosis 1 rapidly concludes. Anaphase 1 and telophase 1 proceed quickly, followed – without an intervening period of DNA synthesis – by the second meiotic division.
Anaphase 1
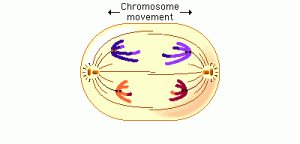
In anaphase 1, the microtubules of the spindle fibers begin to shorten. As they shorten, they break the chiasmata and pull the centromeres toward the poles, dragging the chromosomes along with them. Because the microtubules are attached to kinetochores on only one side of each centromere, the individual centromeres are not pulled apart to form two daughter centromeres, as they are in mitosis. Instead, the entire centromere moves to one pole, taking both sister chromatids with it. When the spindle fibers have fully contracted, each pole has a complete haploid set of chromosomes consisting of one member of each homologous pair. Because of the random orientation of homologous chromosomes on the metaphase plate, a pole may receive either the maternal or the paternal homologue from each chromosome pair. As a result, the genes on different chromosomes assort independently; that is, meiosis 1 result in the independent assortment of maternal and paternal chromosomes into the gametes.
Telophase 1
By the beginning of telophase 1, the chromosomes have segregated into two clusters, one at each pole of the cell. Now the nuclear membrane re-forms around each daughter nucleus. Because each chromosome within a daughter nucleus replicated before meiosis 1 began, each now contains two sister chromatids attached by a common centromere. Importantly, the sister chromatids are no longer identical, because of the crossing over that occurred in prophase 1. Cytokinesis may or may not occur after telophase 1. The second meiotic division, meiosis 2, occurs after an interval of variable length.
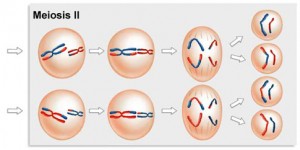
The Second Meiotic Division
After a typically brief interphase, in which no DNA synthesis occurs, the second meiotic division begins.
Meiosis 2 resembles a normal mitotic division. Prophase 2, metaphase 2, anaphase 2, and telophase 2 follow in quick succession.
Prophase 2. At the two poles of the cell the clusters of chromosomes enter a brief prophase 2, each nuclear envelope breaking down as a new spindle forms.
Metaphase 2. In metaphase 2, spindle fibers bind to both sides of the centromeres.
Anaphase 2. The spindle fibers contract, splitting the centromeres and moving the sister chromatids to opposite poles.
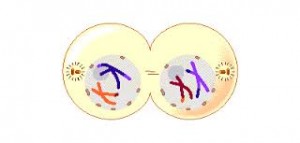
Telophase 2. Finally, the nuclear envelope re-forms around the four sets of daughter chromosomes.
The final result of this division is four cells containing haploid sets of chromosomes. No two are alike, because of the crossing over in prophase 1. Nuclear envelopes then form around each haploid set of chromosomes. The cells that contain these haploid nuclei may develop directly into gametes, as they do in animals. Alternatively, they may themselves divide mitotically, as they do in plants, fungi, and many protists, eventually producing greater numbers of gametes or, as in the case of some plants and insects, adult individuals of varying ploidy.
During meiosis 1, homologous chromosomes move toward opposite poles in anaphase 1, and individual chromosomes cluster at the two poles in telophase 1. At the end of meiosis 2, each of the four haploid calls contains one copy of every chromosome in the set, rather than two. Because of crossing over, no two cells are the same. These haploid cells may develop directly into gametes, as in animals, or they may divide by mitosis, as in plants, fungi, and many protists.
Chapter 2 : Replication
1) Definition
The DNA replication is one of the key mechanisms of cell cycle. It is a highly regulated biological process in which new synthesis and repair mechanisms ensure the integrity of the genome.
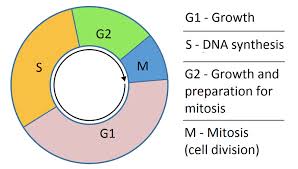
S phase or replication phase follows the G1 phase during which the cell synthesizes all the elements necessary for this replication. The DNA duplication takes about 8 hours.
2) substrates ot the replication
2.1. template strand
The double helix of DNA is separated into two DNA strands. Each strand is then used as template for the synthesis of a new strand.
Replication produces 2 hybrid molecules formed from a parent strand and a daugther strand : replication is semi-conservative.
2.2. Nucleotides
- Nucleotides: dATP, dCTP, dTTP dGTP providing both:
the substrate: the nucleoside monophosphate,
Energy for linking nucleotides between them.
2.3. Enzymes
a) there are several DNA polymerases according to their replicative activity:
– alpha: Start Replication
– delta : replication of the nuclear genome
– Gamma: replication of the mitochondrial genome
– beta: DNA repair
– epsilon: replication of telomeres
b) other proteins are also required during replication
3) Mechanism of replication
3.1. origines of replication
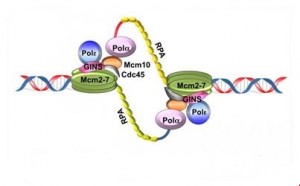
In eukaryotes, replication is initiated at many replication origins called OriC, unlike prokaryotes in which there is a single origin of replication.
Eukaryotic cells must replicate their entire genome for each cell cycle. To carry out this process in a short time, the initiation of replication is made at several OriC or “replication origins” along the chromosome (about 10000) in independent units of replication called replicon (30-300 bp).
In eukaryotes, the best characterized replication origins are those of the yeast. They are named for Autonomously Replicating Sequence ARS. These ARS regions rich in AT, possess fixation sites for the ORC complex.
Then other proteins capable of unwinding the DNA double helix are recruited (by activation of helicases).
3.2. Activation of replication origins
3.2.1. pre-replication complex formation
Replication origins are recognized by protein complexes “ORC” (origin recognizing Complex ) formed of six subunits, each of which is fixed on each replication origin.
Following this fixation, two other factors, Cdc6 / Cdc18 and CDT1, join the first complex to recruit six protein complexes (MCM2, 3,5,6,7,8 )
3.2.2.Activation of the pre-replication complex
1) Activation of CDK
2) phosphorylation / activation of proteins including helicases (Mcm2-7)
3) helicases unwind the DNA double helix and separate the two strands.
The unwinding of the DNA and separating of the two strands exposes single-stranded DNA regions, which are stabilized with protein RPA (replication protein A) and accessible to enzymes and proteins required for replication.
4) Recruitment of DNA polymerases and other proteins
3.3. DNA polymerases
DNA polymerases (or deoxynucleotidyl transferase) are the enzymes responsible for the polymerization of the nucleotides in DNA replication. Prokaryotic DNA polymerases are 3 types (I, II and III) and eukaryotic DNA polymerases of 5 types (α, β, δ, ε and γ).
3.3.1 DNA polymerase α
The DNA polymerase α (α- primase) synthesizes primers which is a short RNA segment (10 base pairs). These short RNA fragments are then elongated by a DNA fragment (20 to 40 nucleotides long). An RNA-DNA primer is created.
3.3.2. DNA polymerase δ
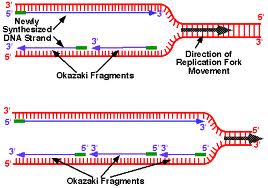
The synthesis of DNA is always in the 5 ‘to 3’, so this requires the presence of a leading strand (primary) which is the strand read in the fork direction and a lagging strand (secondary) is the strand read in the opposite direction of the fork and is said discontinuous strand. There is talk of semi-discontinuous replication.
A complex RF-C / PCNA (replication factor C/ Proliferating cell nuclear antigen) is fixed on the 3’OH end of the primer RNA / DNA and dissociates DNA polymerase α of the DNA template, leaving instead the DNA polymerase δ which recognizes the complex RFC / PCNA and be responsible for the synthesis of continuous strand.
On the strand with discontinuous synthesis, DNA polymerase α-primase which was previously dissociated, restarts the synthesis of a new primer RNA / DNA. On this DNA strand, the enzyme synthesizes multiple small DNA fragments in the prologation of primers, called “Okazaki fragments” (along approximately 1000 to 5000 nucleotides).
As the progression of the replication goes on :
helicase unwind the DNA double helix and topo- isomerase reduces twisting of the DNA upstream of the forks.
3.3.3. DNA ligase
It reconstructs the phosphoester bond between the 3′-OH carbon and phosphate-5′ of two adjacent nucleotides in a DNA strand.
It is also involved in many DNA repair processes.
3.4 The topoisomerases
Type I
– Performs a cut on one of the strands of DNA.
– Transition
– Release the twist.
– No ATP necessary.
Type I I
– Performs a cut on the two strands of DNA.
– Requires ATP.
Chapter 1 : TRANSCRIPTION
The central dogma of molecular biology
The central dogma is the classic sequence of events :
DNA produces RNA by transcription and RNA produces proteins ( structural proteins and Enzymes ) by translation .
Transcription: making of RNA (taking the information of DNA and copying the information to (transitional form) RNA which is unstable.
Translation: is the act of synthesizing a protein from the information in the RNA.
Against central dogma:
-Enzymes can be also certain forms of RNA.
-Now we can take RNA and make DNA from that.
-There are enzymes that can make RNA from RNA.
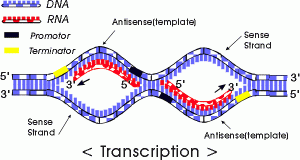
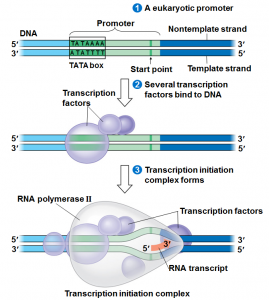
We will begin by transcription in prokaryotes.Typically in prokaryotes the transcription produces an RNA that encodes for more than one protein. Transcription starts at a promoter and make RNA at the same direction that DNA polymerase do for DNA (5’→3’) direction. RNA polymerase which recognizes promoter and does transcription (promoter is defined the site that bound by RNA polymerase and where transcription starts).
There are 2 parts to a promoter : there are so called -10 sequences and so called -35 sequences. What the -10 and -35 refer to is the distance in base pairs from the first base of DNA that is copied into RNA.These sequences are highly characteristic of all genes in the case of -10 of the classes and the genes on the -35 sequences.
The -35 sequence is recognized by a protein (a family of proteins referred to) as sigma factors, these are regulatory proteins. Bacterial RNA polymerases are formed by 4 different subunits (2 copies of big subunits called α and copies of β and β’) ⇒ α2ββ’.
α2ββ’ is the holoenzyme and then σ factor is a regulatory subunit and comes in many varieties. There are many σ factors ,the key thing with σ is that they are specific to particular promoters ,examples :
1)Heatshok
2) sporulation
There are many factors, the key thing with factors is that they are specific to particular promoters.
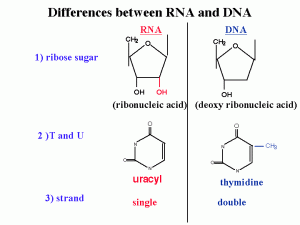
Differences between DNA and RNA :
1)2’ OH is special for RNA
DNA is deoxy. This OH group makes RNA unstable and vulnerable by enzymes or bases or different kinds of biophysical conditions. So RNA is designed to be unstable. Why RNA must be unstable (the earliest forms of life used RNA as a genetic material). Why we use an unstable molecule? probably it’s for the control of gene expression.
2) uracyl instead of thymidine
3) no primer required for the synthesis of RNA from RNA
4) synthesized in 5’→3’ direction (the chemistry involved is identical to DNA polymerase) :Initiation-elongation-termination
Step 1 :closed promoter complex
Step 2: formation of open promoter complex which is 17 bases pairs (normally there are 10 bases pairs by turn of helix) So 17 is almost 2 turns of helix in this situation, RNA polymerase start to synthesize RNA.
How we can confirm that? by DNA foot printing….