A chiral agent is always necessary to differentiate the enantiomers. The most used methods to determine the ee are
- the GC with a chiral stationnary phase,
- HPLC with a chiral stationnary phase,
- NMR with chiral lanthanides.
Gazeous chromatography
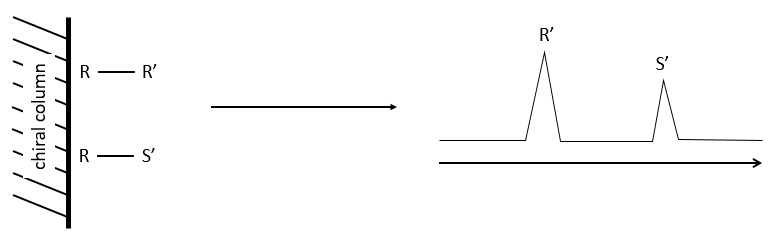
The stationnary phase of the column is chiral and only composed of one enantiomer (for instance the R configuration). When a racemic melange passes through the column, the enantiomers R’ and S’ have a different interaction with the chiral stationnary phase with which they form diastereoisomeric complexes.
The main interactions can be H bonds, sterical interactions or dipole-dipole interactions. As the interactions have not the same strength, the enantiomers R’ and S’ have a different retention time and exit the column separately, giving two peaks.
The advantages of this method is that one the condition of experimentation are setted up, the analysis is fast and has a high sensibility. It can detect an enantiomer even if its proportion in the solution is about 0.5%. Moreover, the analysis separates the species composing the sample and we can select the portion of the solution that we want to analyse next.
When we use this method, the racemic melange has first to be tested to be sure that the enantiomers can be separated and that the ratio of the areas of the peaks is 50:50.
NMR
This technique is not as sensible as the GC but it is very fast and cheap.
Normally, enantiomers have the same NMR spectrum because the interatomic distances are identical for the two enantiomers. Diastereoisomers have spectra that can be distinguished. To distinguish two enaintiomers we will thus transform them into diastereoisomers.
To do so, we use two types of chiral reactants.
- chiral agent of derivatisation
- chiral lanthanides
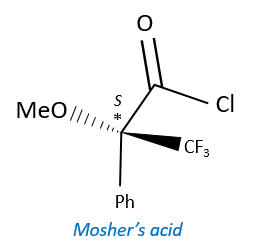
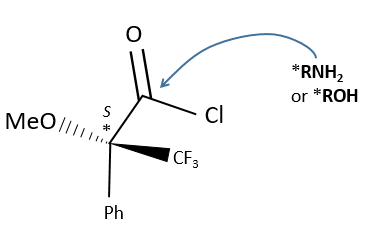
A chiral agent of derivatisation is a chiral compound optically pure that interacts with the enantiomers. The most used one is the Mosher’s acid (α-methoxy-α-trifluoromethylphenylacetic acid (MTPA)).
This reactant forms a covalent liaison with the enantiomer to form diastereoisomers with an important difference of chemical displacement. The ratio between the areas gives the enantiomeric excess.
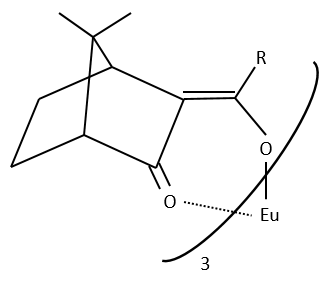
The method with chiral lanthanides is more used than the method with a chiral agent of derivation.
Lanthanides allow a widening of the NMR spectrum by the displacement of the signals towards the low fields. The salt of lanthanide is added bit by bit to the sample to observe the separation of the signals. They form a complex with the enantiomer without covalent liaison but still give an important separation of chemical displacement.
The disadvantages of this method is that it takes more time (the salt is added slowly) and that the salt is expensive.